Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Sugar Link Calms Inflammation
Modified antibody could underpin better autoimmune therapies
by Carmen Drahl
April 21, 2008
| A version of this story appeared in
Volume 86, Issue 16

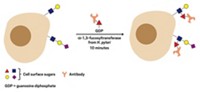
Researchers have identified a specific sugar linkage on an antibody that is responsible for the antibody's anti-inflammatory activity, a discovery that may lead to improved treatments for autoimmune diseases (Science 2008, 320, 373).
Physicians sometimes soothe inflammation in patients with lupus or rheumatoid arthritis with an antibody treatment called intravenous immunoglobulin G. IVIG is actually a mixture of antibodies modified with different branched sugar chains, and patients need high doses to relieve inflammation. Immunologist Jeffrey V. Ravetch from Rockefeller University and colleagues from the University of New Hampshire and the Scripps Research Institute have now shown that anti-inflammatory activity depends on how terminal sialic acid sugars in IVIG are linked to the next sugar in the chain. The scientists used that information to generate a synthetic antibody that relieves inflammation in arthritic mice at a dose 30 times lower than IVIG.
In previous work, Ravetch's team showed that only a tiny fraction of IVIG—the portion containing polysaccharides capped with sialic acid—is biologically active. But sialic acid is known to attach to sugar chains in different ways, Ravetch says. The new study sought to determine which among those possibilities led to anti-inflammatory activity.
The team used mass spectrometry and enzymes capable of selectively creating or clipping specific sugar linkages to demonstrate that α2,6-linked sialic acids in IVIG are responsible for the treatment's anti-inflammatory powers. Ultimately, the team made a synthetic human antibody tipped with only α2,6-linked sialic acid and showed that it was highly effective at calming inflammation in arthritic mice.
This work "presents an elegant example of the importance of precise protein glycosylation for optimal activity of therapeutic glycoproteins," comments Geert-Jan Boons of the Complex Carbohydrate Research Center at the University of Georgia, Athens.
Interestingly, Ravetch notes, antibodies traditionally trigger inflammation, but a specific change makes IVIG soothe instead. "What's remarkable about this antibody is the process of posttranslational modification that completely changes the biological activity," he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter