Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Telomerase Component's Structure Solved
Catalytic subunit forms doughnut
by Celia Henry Arnaud
September 8, 2008
| A version of this story appeared in
Volume 86, Issue 36

IN A MAJOR ADVANCE toward understanding a key biomolecule, scientists have solved the structure of the catalytic subunit of telomerase, the enzyme that maintains the length and integrity of telomeres, the DNA sequences at the ends of chromosomes. Telomerase activity usually declines as a natural part of aging, limiting cell division, but it reactivates in cancer, allowing cancer cells to continue dividing.
Elizabeth Blackburn, a biochemistry professor at the University of California, San Francisco, and one of the codiscoverers of telomerase, says, "The new structure of the protein portion of the ribonucleoprotein enzyme telomerase is an important step along the way to the ultimate understanding of this enzyme and its potential exploitation for medical purposes." Telomerase is a potential drug target for treating cancer and some other diseases, and for mitigating effects of aging.
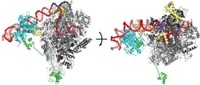
Telomerase consists of a catalytic protein subunit (telomerase reverse transcriptase, or TERT) and an RNA component that provides the template for the DNA repeat sequences that the enzyme adds to the ends of chromosomes. Assistant professor Emmanuel Skordalakes, research assistant Andrew J. Gillis, and lab assistant Anthony P. Schuller of Wistar Institute, in Philadelphia, solved a 2.71-?? crystal structure of TERT from the red flour beetle, Tribolium castaneum (Nature, DOI: 10.1038/nature07283).
"The paper is exciting because it's the first view of the entire catalytic subunit of telomerase," says Thomas R. Cech, president of Howard Hughes Medical Institute. Cech's group has also been working on the structure of telomerase at the University of Colorado, Boulder. "Many labs have tried to purify and crystallize various TERTs, and most gave up in despair. The red flour beetle to the rescue!" Cech says.
Skordalakes acknowledges that the breakthrough came when they found an organism that allowed them to express large amounts of the protein.

TERT contains three domains—an RNA-binding domain, a reverse transcriptase domain, and a so-called thumb domain. These domains adopt an unexpected doughnutlike structure. The business portions of the protein, including the nucleic acid-binding sites and the catalytic site, are located inside the doughnut hole, a cavity that is about 26 Å wide and 21 Å deep. The cavity is the perfect size to hold double-stranded nucleic acid molecules that are seven or eight base pairs long, about the size of a telomeric repeat, Skordalakes says.
To better understand how TERT's ring associates with RNA-DNA hybrids to form the complex that catalyzes telomere elongation, Skordalakes, Gillis, and Schuller carried out computational modeling on complexes of RNA-DNA hybrids with HIV reverse transcriptase, TERT's closest structural homolog. Skordalakes was excited that the models showed that the RNA-DNA hybrids fit perfectly inside the ring.
"We'd love to have a structure of the whole complex" of TERT with telomerase's RNA component, he says. "That's our next goal."
Blackburn agrees that the story is far from over. Only a small part of the telomerase RNA component serves as the template, she says. The much larger remainder "nestles into the TERT protein" and must collaborate with TERT to make telomeres, she says. "An important challenge still lies ahead: to figure out how that telomerase RNA-protein collaboration actually works," she says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter