Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Safety
FDA’s Foreign Inspection Program Needs Overhaul
by Glenn Hess
November 3, 2008
| A version of this story appeared in
Volume 86, Issue 44
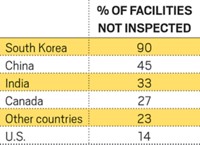
Better data management and more inspections are needed to strengthen FDA’s foreign drug facility inspection program, according to congressional investigators. The Government Accountability Office says the agency isn’t sure how many foreign establishments produce drugs for the U.S. market (GAO-08–970). Using a list of 3,249 plants FDA developed in 2007 to prioritize foreign inspections, GAO estimates that FDA inspects only about 8% of facilities outside the U.S. each year. “At this rate, it would take FDA more than 13 years to inspect these establishments once,” GAO notes. Pharmaceutical plants in the U.S. are inspected on average every 2.7 years. “This report confirms that we have reason to be concerned about the safety of imported drugs,” says House Energy & Commerce Committee Chairman John D. Dingell (D-Mich.). “Foreign inspections are alarmingly low.” The report also indicates that FDA is failing to promptly conduct follow-up inspections after serious violations—such as product impurities or record-keeping problems—are found in foreign facilities. From 2002 through 2007, FDA issued 15 warning letters to foreign drug producers but reinspected only four of the facilities, the report notes.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter