Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
DNA-Guided Crystallization
Selective biochemical interactions organize metal nanoparticles
by Mitch Jacoby
January 31, 2008
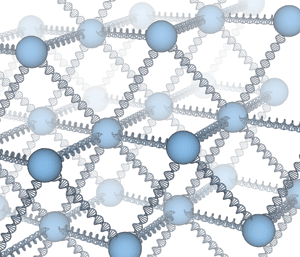
A new method uses strands of DNA to guide nanoparticles into crystals.
DNA's knack for selective pairing of complementary strands can drive metal nanoparticles to assemble into crystals, according to two new studies in Nature (2008, 451, 549 and 553). The investigations demonstrate a strategy for producing novel self-assembling materials by chemically modifying nanoscale building blocks.
In both studies, researchers grafted numerous strands of DNA onto gold particles with diameters in the range of 10 to 15 nm. The DNA-based tethers were composed of various segments and designed such that a relatively short section at the end of each strand would pair with a complementary section on another DNA-adorned nanoparticle. The pairing process—or hybridization reaction—forms DNA double helices and guides the tagged nanoparticles to line up in an orderly fashion in three dimensions, thereby forming micrometer-sized crystals.
The investigations differed somewhat in their focus. In one case, a team led by Oleg Gang at Brookhaven National Laboratory explored the temperature dependence of DNA-induced crystallization. They report that crystals form reversibly during heating and cooling cycles and that crystallization is affected by the length of a spacer segment within each DNA tether. Particles with long spacers (35 or 50 nucleobases) crystallize readily, whereas those with short or rigid bases do not crystallize.
Meanwhile, at Northwestern University, a team led by chemists Chad A. Mirkin and George C. Schatz probed the range of crystal structures that can be formed from a single type of nanoparticle. They find that when only one type of tether is used, the particles crystallize with a face-centered-cubic structure. But by combining nanoparticles with one type of tether with particles having a tether with a different DNA sequence, the nanoparticles form body-center-cubic crystals.
Commenting in the same issue of Nature, University of Pennsylvania chemical engineering professor John C. Crocker notes that the method might be used with particles of nearly any material to make novel composites endowed with unusual electronic and optical properties.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter