Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
New Motions For Molecular Machinery
Hydrogen-bonded crystalline assembly undergoes three distinct temperature-induced motions: translation, rotation, and tilting
by Stephen K. Ritter
January 31, 2008

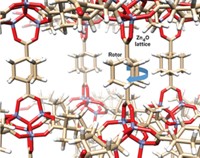
Macromolecules that can undergo a range of motions when stimulated by light or heat have been touted for several years as potential components of tiny machines in future nanoscale devices. One such molecular assembly, reported by Leonard R. MacGillivray, Anatoliy N. Sokolov, and Dale C. Swenson at the University of Iowa, is the first example of an organic solid in which the molecules can undergo three correlated motions, in this case functioning like a set of rack-and-pinion gears that convert linear motion into rotary motion (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.0706117105).
MacGillivray's group used its previously developed templating strategy to cocrystallize an alkyl phenol (4-hexylresorcinol) with a diene (all-trans-2,5-bis[4-ethenylpyridyl]thiophene). In this assembly, held together by four N∙∙∙H–O hydrogen bonds, two diene molecules are sandwiched between two phenol molecules.
Upon cooling, the assembly's molecules reorganize, which the researchers observed by X-ray crystallography. The hexyl groups, serving as the rack, expand and contract. There is a subsequent 180° crankshaft rotation about one of the dienes' double bonds, serving as the pinion gear. In combination with these motions, the assembly???s aromatic rings tilt. The collective molecular motions are reversible upon heating.
UCLA's Miguel A. Garcia-Garibay, also a molecular machinist, comments that "MacGillivray and coworkers illustrate how a temperature-induced transition between different crystal phases may offer a deep insight into the type of complex processes taking place within the bulk of single-crystalline specimens."
Future studies will likely address the rate of these motions as a function of temperature, and it might be possible to take advantage of small thermal fluctuations around a critical temperature to operate the machinery, Garcia-Garibay adds. "But more important, MacGillivray and coworkers provide a new perspective on crystalline molecular machinery, and we can expect that new developments may come at an accelerated pace," he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter