Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
How An Anti-HIV Drug Sidesteps Resistance
Inhibitor modifies its shape to accommodate changes in enzyme conformation
by Stu Borman
February 7, 2008
As HIV reverse transcriptase (gray, orange, and red) changes its shape through mutation, TMC278 (green and blue) follows the changes (yellow and blue) and stays bound.
Human immunodeficiency virus is usually pretty good at mutating, thereby developing resistance to antiretroviral drugs. Agents targeting the virus's reverse transcriptase enzyme sometimes lose their efficacy when the enzyme mutates, but the inhibitor TMC278 (rilpivirine) tends to remain effective. Researchers have now obtained crystal structures that show that the drug shifts its shape, enabling it to hold on to mutated versions of the enzyme, an ability that might be incorporated into the design of other drugs.
The diarylpyrimidine TMC278 "is a highly promising compound" that has shown efficacy against a broad range of drug-resistant HIV variants, says virologist John M. Coffin of Tufts University, who did not participate in the crystallographic study. The compound is entering Phase III clinical trials sponsored by Tibotec Pharmaceuticals. The related diarylpyrimidine TMC125 (etravirine, or Intelence) was approved by FDA last month for treatment of antiretroviral drug-resistant HIV infections.
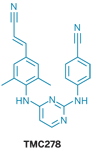
To learn why TMC278 inhibits HIV reverse transcriptase so persistently, a research team has obtained high-resolution crystal structures of the native enzyme and two key drug-resistant mutants, each bound to TMC278. The structures show that the inhibitor's compactness and flexibility enable it to adjust its conformation to accommodate mutational changes in the enzyme binding pocket (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.0711209105).

The work was carried out by professor of chemistry and chemical biology Eddy Arnold of Rutgers University, whose group helped discover TMC278; Aaron J. Shatkin, professor and director of the Center for Advanced Biotechnology & Medicine, Piscataway, N.J.; and their coworkers, including Kalyan Das and Joseph D. Bauman. The researchers note that even as the enzyme's binding pocket adjusts its shape through mutation, it remains tightly wrapped around TMC278, which is flexible enough to make complementary conformational changes.
Arnold, Shatkin, and coworkers note that such mutual adaptability suggests the desirability of a more dynamic approach to drug design than is used conventionally. Shape modifications in protein targets and possible corresponding conformational adjustment in drugs should both be considered in the rational design of agents "intended to be broadly effective against targets that readily mutate and develop drug resistance," the researchers write.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter