Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
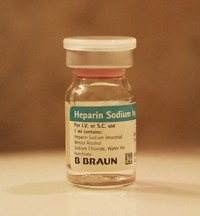
Heparin Contaminant Is Chondroitin Sulfate
FDA identifies adulterant in drug lots associated with adverse reactions
by Jyllian Kemsley
March 19, 2008

FDA announced on March 19 that in collaboration with academic labs it has identified a contaminant in heparin linked to severe allergic reactions to the drug. The contaminant is chondroitin sulfate (CS), which like heparin is a variably sulfated glycosaminoglycan. CS is found in cartilage and commonly used in the U.S. as a dietary supplement to treat arthritis.
In particular, the CS found in heparin was chemically modified with additional sulfate groups. Such oversulfated, or hypersulfated, CS is not known to exist biologically, and thus it would not have been incorporated into heparin products as an artifact of purification, said Janet Woodcock, director of FDA's Center for Drug Evaluation & Research. Unlike typical CS, oversulfated CS mimics heparin in standard regulatory tests, including potency assays. There is little clinical information on the biological properties of oversulfated heparin, Woodcock added.
FDA is continuing to investigate whether the oversulfated CS was added to heparin accidentally or intentionally, Woodcock said. She noted that CS is abundantly available and inexpensive. CS is commonly isolated from cow and pig cartilage.
The oversulfated CS was found in heparin active pharmaceutical ingredient (API) lots from Scientific Protein Laboratories' (SPL) Changzhou plant in China, as well as the crude heparin used to make the API. In some samples as much as 50% of the API was oversulfated CS. SPL supplied Baxter Healthcare with heparin API. Baxter has recalled its heparin products, as have three Japanese companies—Terumo, Otsuka Pharmaceutical, and Fuso Pharmaceutical Industries—that used heparin API supplied by SPL. German company Rotexmedica GmbH, a unit of the French company Groupe Panpharma, has also recalled heparin sourced from China, although from different suppliers.
FDA and the U.S. Pharmacopeia, which describes itself as the "public standards-setting authority for all prescription and over-the-counter medicines, dietary supplements, and other healthcare products manufactured and sold in the United States," have agreed to expedite development of tests to detect oversulfated CS in heparin. The tests will most likely use nuclear magnetic resonance spectroscopy and capillary electrophoresis, Woodcock said.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter