Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Novel Fenestranes In A Snap
Cyclization-oxidation cascade stitches up polycyclic structures
by Bethany Halford
March 25, 2008

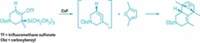
Using a one-pot procedure, chemists in France have developed a simple synthesis of the complex structures of [4.6.4.6]fenestradienes and [4.6.4.6]fenestrenes. The efficient method provides rapid access to these unusual molecular scaffolds, which could be used as materials, ligands for catalysis, and pharmaceuticals.
Named after the Latin word for window, the fenestrane family of molecules features a windowpane-like structure in which four rings share a quaternary carbon. Accessing these polycycles' strained structures poses a challenge for synthetic chemists. Using a trienyne as their starting point, Jean Suffert, Catherine Hulot, and Gaellë Blond of Louis Pasteur University of Strasbourg were able to design a cyclization cascade that assembles a [4.6.4.6]fenestrane skeleton with good yields (J. Am. Chem. Soc., DOI: 10.1021/ja800691c).
In the synthesis' key step, a nickel catalyst partially reduces the trienyne's triple bond. The researchers believe that the tetraene generated in this reaction undergoes an 8π-conrotatory electrocyclization followed by a 6π-disrotatory electrocyclization to form a [4.6.4.6]fenestradiene. This highly reactive compound readily oxidizes in air to form a [4.6.4.6]fenestrene. "The high yields and the limited number of steps of the reaction sequence render this process very attractive for the synthesis of new fenestranes," write the authors.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter