Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Multilayer Metal-Organic Films Go 3-D
Metal-ligand coordination and π???π interactions provide unprecedented structure to layered molecular assemblies
by Stephen K. Ritter
April 10, 2008

By taking advantage of metal-ligand binding in one direction and intermolecular interactions in a different direction, Milko E. van der Boom at the Weizmann Institute of Science, Rehovot, Israel, and coworkers have devised a fundamentally new approach to building functional multilayer thin films on a substrate. As van der Boom explained during a Division of Inorganic Chemistry presentation at the ACS national meeting in New Orleans this week, the three-dimensional molecular assemblies could eventually be used in a variety of chemical-sensing or molecular-switch applications.
The method is ???a very creative hierarchical combination of molecular interactions to craft crystallike ordered assemblies,??? commented John A. Gladysz, a chemistry professor at Texas A&M University.
Gladysz, whose work includes using metal coordination to build molecular gyroscopes and related helical metal-organic systems, noted that all sorts of monofunctional monolayers exist, including some that are cross-linked in various ways. Multilayer films also can be made by stacking monolayers on top of each other, he added. But those systems are not modular in nature nor are they continuous molecular assemblies like those made by van der Boom's group.
???This is a new concept that really expands the toolbox of reactions that chemists can use to build multilayer assemblies,??? Gladysz observed.
The 3-D assemblies came about as an extension of van der Boom's earlier work on monolayers containing metal complexes. Those traditional thin-film systems consist of redox-active osmium and/or ruthenium bipyridine complexes tethered to a silicon substrate via siloxane chains, van der Boom explained.
The monolayers are proving to have potential as part-per-million level chemical sensors to detect water, carbon monoxide, nitrogen oxides, and various metal ions, he said. Van der Boom believes they could be used as moisture detectors for organic solvents and fuels or to detect toxic aqueous metal ions such as Cr6+ (J. Am. Chem. Soc. 2008, 130, 2744).
In another case, van der Boom's group created a system in which monolayers on separate substrates use a metal ion to transfer electrons back and forth (Angew. Chem. Int. Ed. 2008, 47, 2260). These monolayer artificial communication systems could serve as chemical sensors or possibly even as information storage devices, he said.
Van der Boom's group more recently began to explore making multilayers by linking organic groups and metal atoms into oligomers, which led to the novel 3-D systems (J. Am. Chem. Soc., DOI: 10.1021/ja800563h).
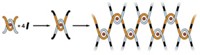
The researchers first create a standard monolayer by treating a silicon substrate with a phenylene chromophore terminated on both ends by a pyridine ring. One of the pyridines sports a siloxane substituent, which attaches the phenylene to the substrate.
As a second step, the team exposes the phenylene layer to a PdCl2 solution in order to coordinate palladium atoms to the free pyridine nitrogen atoms. The bound palladium atoms then serve as the foundation for the assembly's second layer.
In the final maneuver, the researchers introduce anthracene functionalized with two vinylpyridine units. One vinylpyridine coordinates to the exposed palladium atoms, leaving the second pyridine free to accommodate the next round of palladium. Further iterations of anthracene and palladium create a set of aligned, wirelike oligomers attached to the substrate.
But van der Boom's choice of anthracene as a ligand provides a unique twist. The anthracene units on adjacent chains align their three fused rings face-to-face as a result of aromatic π???π interactions. These sterically demanding secondary forces lock in lateral stability to the metal-organic chains, he explained, resulting in a highly ordered molecular framework that he and his colleagues are currently developing into a host of applications.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter