Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Protective Protein
Membrane enzyme maintains tuberculosis bacterium's pH
by Carrie Arnold
July 24, 2008

An enzyme in the membrane of the tuberculosis bacterium, Mycobacterium tuberculosis, affects the microbe's virulence by controlling its internal pH level, say researchers who made the discovery. The protein is a potentially important new target for TB treatments, the researchers say.
The World Health Organization reports that TB is a major cause of death in many developing nations and estimates that one-third of the world's population is infected with M. tuberculosis. However, only 10% of infected people will become ill with TB. After infection, the TB bacterium evades the immune system and can lie dormant for decades.
Although scientists have long understood how and where M. tuberculosis survives in the body, they remained uncertain of the precise mechanisms used by the bacterium to escape destruction. Normally, when an immune system macrophage encounters a bacterium, it engulfs the invader, ultimately acidifying and destroying it. Yet M. tuberculosis can survive inside a macrophage for decades, despite this drop in pH.
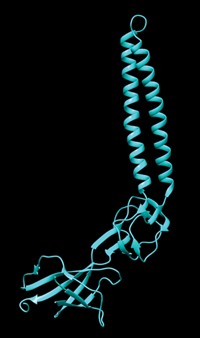
A team of researchers at Weill Cornell Medical College of Cornell University, led by Sabine Ehrt and Carl F. Nathan, has found that the membrane-associated serine protease Rv3671c is the enzyme responsible for this ability to survive in low pH (Nat. Med., DOI:10.1038/nm.1795). Ehrt, Nathan, and coworkers found that strains of M. tuberculosis lacking Rv3671c were unable to maintain their pH balance.
The Cornell team also demonstrated that the virulence of M. tuberculosis depends in part upon the functioning of this enzyme. TB bacteria lacking a functional Rv3671c enzyme were unable to cause disease in mice, the researchers found. They hypothesize that without the functional serine protease, the bacteria are unable to stop a lethal influx of protons.
David R. Sherman, associate professor of pathobiology at the University of Washington, comments that he's not sure inhibiting Rv3671c will "pay off against persistent bacteria and shorten the course of therapy, but it is definitely a very interesting and worthwhile avenue to pursue."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter