Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Ship-In-A-Bottle Catalysis
Zeolite-type frameworks serve as robust catalyst platforms
by Mitch Jacoby
September 11, 2008

Catalyst molecules immobilized inside zeolite-like metal-organic-framework (ZMOF) compounds outperform the same molecules when used in solution or when attached to other types of solids, according to a new study (J. Am. Chem. Soc., DOI: 10.1021/ja804703w).
The investigation, which focuses on metalloporphyrin-based catalysts, demonstrates a general strategy for enhancing a catalyst's activity. The work also broadens the growing range of chemical uses for metal-organic frameworks (MOFs), which are crystalline, porous compounds composed of metal ions connected by organic linkers (C&EN, Aug. 25, page 13).
Metalloporphyrins have been widely used to catalyze epoxidations, hydroxylations, and other types of reactions. But as solution-phase catalysts, these heterocyclic compounds often suffer from limited lifetimes as a result of destructive reactions. For example, autodimerization of porphyrins results in bridged structures that essentially block access by reagents to catalytically active sites.
Previously, researchers tried sidestepping these problems by immobilizing the porphyrins on porous silicates and other types of inorganic solids. That approach, however, led to assemblies with low concentrations of weakly held catalysts that were often leached from the solids.
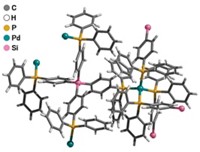
Now, University of South Florida chemistry professor Mohamed Eddaoudi, grad student Mohamed H. Alkordi, and coworkers offer a new approach: Immobilize the metalloporphyrins in a ZMOF. In particular, they have shown that the anionic In-imidazoledicarboxylate ZMOF compound firmly anchors cationic metalloporphyrins inside the structure's large cages. They prepared the ZMOF crystals from a mixture of ZMOF precursors and porphyrins; in effect, the cages grew around the catalysts. In a postassembly step the porphyrins were metallated. The overall ZMOF structure consists of interconnected cages that are linked through windows that are too small to allow the porphyrins to escape but are roomy enough to allow many kinds of reactants and products to diffuse in and out of the cages.
"The porphyrins are stuck in prison," Eddaoudi says. Not only are they held firmly in the MOF structure, they are also isolated from one another and thus cannot dimerize and deactivate.
Catalysis tests show that a ZMOF containing a Mn-porphyrin catalyzes cyclohexane oxidation to a mixture of cyclohexanone and cyclohexanol in greater than 90% yield, the team reports. They observed no activity for all control reactions. The catalyst retained its activity, crystallinity, and other properties even after being recycled and reused a dozen times, they say. Now the group is studying alkene epoxidation and other reactions.
Northwestern University chemistry professor Joseph T. Hupp remarks that MOF-based catalysis is an area of great promise with little fulfillment thus far. He says the Eddaoudi group's "zeolite ship-in-a-bottle" approach to MOF-mediated catalysis is "very clever." Hupp predicts that follow-up studies of these complexes will reveal other advantages of this catalytic strategy.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter