Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Polyhedral Boranes: Chemistry For The Future
by Priestley Medalist M. Frederick Hawthorne
March 23, 2009
| A version of this story appeared in
Volume 87, Issue 12

I AM DEEPLY GRATEFUL for my selection as the Priestley Medalist, an honor that I humbly share with all previous medalists. The 2009 Priestley Medal once again brings boron chemistry into focus: Hermann I. Schlesinger, who worked on small boranes and the borohydride ion, received the medal in 1959; 1979 Chemistry Nobel Laureate Herbert C. Brown, known for his hydroboration research, received the medal in 1981; Roald Hoffmann, a 1981 Chemistry Nobel Laureate, was honored with the medal in 1990 for his contributions to bonding theory; and Robert W. Parry, the 1993 medalist, who also served as American Chemical Society president in 1982, was instrumental in developing borane reactions. These Priestley honorees each made seminal contributions to the chemistry of the fifth element.
In my case, I happened to be in the right place at the right time, and along with my colleagues, I worked to create the fledgling field of modern boron chemistry. As a result, I managed to make a career for the past 50-plus years from the discovery and exploration of polyhedral boranes, carboranes, and their metal-based derivatives.
It all got started in 1956, when I was asked to organize an organometallic chemistry research group for Rohm and Haas at their research laboratory located within the sprawling Redstone Arsenal Army base, in Huntsville, Ala. Up until that time, borane chemistry was primarily the chemistry of the original boron hydrides, which were discovered by Alfred Stock in Germany in the early 1900s. This chemistry was further explored by Schlesinger and his students at the University of Chicago, along with a few other researchers distributed around Europe and the U.S.
Boranes of this type are represented by B2H6, B5H9, and B10H14, to name a few. Simple anions such as BH4- and B3H8- were also well-known. They all have hydrogen-to-boron ratios greater than 1.
These borane species tend to have open structures with low symmetry. Chemists, being clever sorts, some years later came up with descriptive names for these open structures: nido for the nestlike BnHn+4, arachno for the weblike BnHn+6, and hypho for the netlike BnHn+8.
The stabilities of these original boranes were characterized as being variable, ranging from spontaneously catching fire in air, to slow oxidation in air, to reaction with water to form boric acid, B(OH)3, and H2. This hydrolysis with water was characteristic of all the boranes known back in the late 1950s. Thus, the field of borane chemistry at that time was limited to very reactive and unstable borane derivatives with very few useful properties for materials development or in biomedical areas—t hat would come later.
There was, however, a single application that was thoroughly explored: Synthesis of liquid mixtures of ethylated decaborane or methylated pentaborane derivatives that could be handled in air and employed as high-energy fuels for jet engines. This chemistry was carried out in the classified Navy ZIP and Air Force HEF programs—in which our Rohm and Haas lab played a peripheral role—that led to the large-scale production of these inorganic fuels and provided a huge stimulus to borane research.

OF COURSE, the Soviets were working on the same thing. But this effort was quickly abandoned when jet engines meant for high-speed aircraft burning borane fuels failed prematurely, and other technology such as surface-to-air missiles made these aircraft obsolete. The silver lining in this story is that based upon our achievements, we received 1 ton of decaborane, gratis, from the Air Force for the continuation of our exploratory borane research.
Our initial discoveries were serendipitous and stand as tributes to very careful synthesis, separation, and characterization studies. The first vital result involved the reaction of a decaborane derivative with an amine to produce the salt of a strange diamagnetic dianion carefully characterized and formulated as B10H102-. The 11B nuclear magnetic resonance spectrum of this ion proved the presence of two types of boron atoms in a 4:1 ratio.
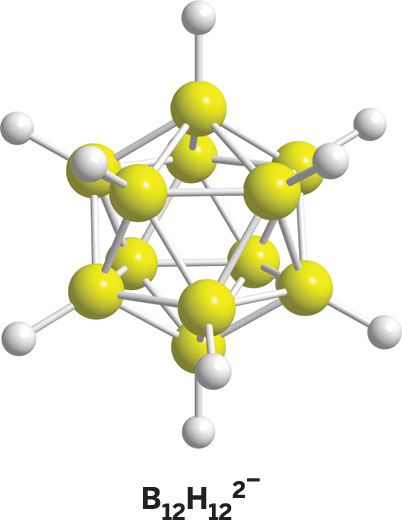
The high symmetry of the ion and its magnetic properties clearly pointed to a symmetrical polyhedral structure (a bicapped Archimedean square antiprism) that was first proposed by William N. Lipscomb's group at Harvard University and by my group—Lipscomb received the 1976 Nobel Prize in Chemistry, in part for his research on the structure and bonding of boranes. This unprecedented BnHn2- formulation proved that a new type of borane dianion containing equal numbers of boron and hydrogen atoms could exist.
Most interesting was the fact that British theoretician Christopher Longuet-Higgins had predicted, on the basis of simple molecular orbital calculations, a closed polyhedral structure for a similar B12H122- ion, whose existence had not yet been reported. Aromaticity of the latter was possible, and it suggested that this icosahedral ion would be very stable if ever made and isolated. The B12H122- ion had actually been shown to exist by Earl L. Muetterties and his colleagues at DuPont, but the discovery remained undisclosed until 1964 for patent purposes.
My group independently isolated, characterized, and reported the existence of B12H122- and its probable icosahedral structure in 1959. Thus, two BnHn2- ions were known from our research, with n = 10 and 12. These two ions can now be synthesized in any amount, and they are common precursors for many new and useful derivatives. Both dianions are extraordinarily stable to heat and hydrolysis.
The complete polyhedral BnHn2− borane dianion series, with n = 6–12, is now known, largely due to Muetterties' DuPont research. These are the so-called closo boranes, named for their closed-cage structures. In terms of organic chemistry, the closo borane dianions are analogs of aromatic hydrocarbons, such as benzene. It was an unusual occurrence that the least stable boranes were discovered before the very stable ones.

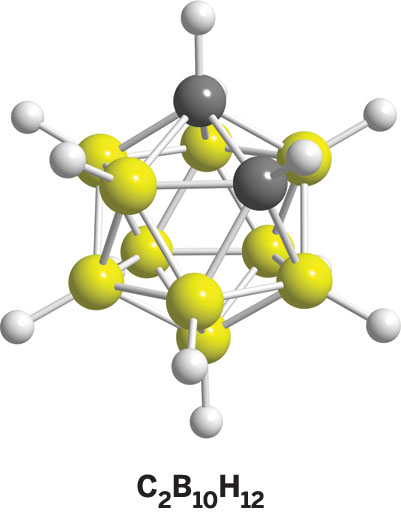
Another fortuitous discovery was made in 1958, when scientists at Reaction Motors (which merged with Thiokol) reported the discovery of very stable organic derivatives having the C2B10H12 formulation. In these carboranes, two carbon atoms have replaced two boron atoms and two electrons in B12H122-. To make a long story short, the closo carborane series C2Bn-2Hn. with n = 5–12 was established through synthesis by my group and others. Variation of the number and position of boron substitutions by carbon in polyhedral boranes turns out to be quite complex, leading to all sorts of isomers. Atoms other than carbon, such as nitrogen, sulfur, and phosphorus, can be introduced in polyhedral borane cages.

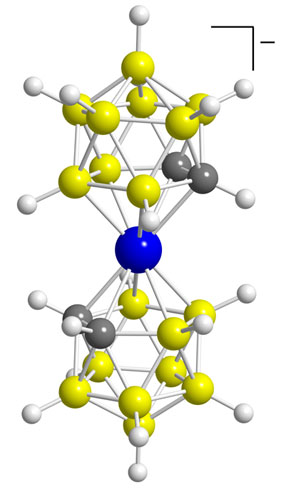
The startling discoveries kept coming. In 1965, after I left Rohm and Haas for the University of California, Riverside, my group reported the synthesis and characterization of the first metallacarboranes. These compounds had been predicted and were deliberately sought, after noting that the cyclopentadienyl ligand (C5H5-) found in metallocenes, such as ferrocene, and the predicted C2B9H112- ion have identical electron counts. In both cases, six π electrons are available on a five-membered ring for delocalized bonding with transition-metal centers. Transition metals such as iron and cobalt form sandwich complexes with two C2B9H112- ions, and the complexes resemble ferrocene's electronic and structural properties. The metallaboranes and metallacarboranes can involve many of the metals across the periodic table.

A general feature of closo polyhedral borane species is their extreme thermal and chemical stabilities, which, if coupled with possible electrochemical properties and photoactivity, suggest many, many applications in all areas of technology ranging from nanomaterials to pharmaceutical design.
For example, in the early 1950s, neutrons to run medical experiments were available at Brookhaven National Laboratory, and neurosurgeon William Sweet of Massachusetts General Hospital used this source for the first series of clinical trials of boron neutron capture therapy (BNCT) for cancer. In this procedure, 10B atoms carried by tumor-targeting compounds are first localized in tumor cells. The next step involves irradiating the 10B nuclei with slow neutrons, leading to the capture of neutrons by 10B nuclei and fission of the resulting unstable 11B to produce 4He and 7Li nuclei plus 2.4 MeV of kinetic energy. These charged atomic nuclei are propelled through the tumor cell, causing ionization and cell death.

Prior to 1958, this procedure had little chance for success because the only boron compounds available were largely boric acid and organic boronic acids, RB(OH)2, which all had low boron content, were toxic, and had poor tumor selectivity. The discovery of Na2B10H10 in our lab opened a new era in the investigation of BNCT because the polyhedral boranes generally have high boron content, are nontoxic, and are chemically stable.
The BnHn2− (n = 10 and 12) ions have been investigated for BNCT by a variety of investigators during the past 25 years with promising results. Leaders in this effort are Patrick R. Gavin of Washington State University and George E. Laramore of the University of Washington School of Medicine.
Our elucidation of carborane chemistry provided a natural bridge between boranes and organic chemistry and led to the synthesis of a wide variety of tumor-specific compounds. Although we have pursued various types of polyhedral borane and carborane species for BNCT over 50 years, we never had access to neutron sources. The efficacy estimations we made with our compounds were limited to biodistribution measurements of boron uptake and selectivity in small animals such as mice.
The opportunity to combine our synthetic research with readily available large-animal experiments prompted me to move two years ago, at this late stage in my career, from UCLA to the University of Missouri, Columbia, where we created the International Institute of Nano & Molecular Medicine. I2NM2, as we call it, was made possible by the University of Missouri School of Medicine and the university's 10-MW nuclear reactor, which is the largest academic-based reactor in the U.S.
We have a newly constructed neutron beam line that allows us to explore in greater detail the medical uses of polyhedral borane chemistry and boron-containing nanomaterials. The research program is just beginning, and we expect important results in the next few months. I feel very fortunate for this opportunity—another example of the right place at the right time.
BUT I WANT TO REVISIT the early times in my life leading up to my formative Rohm and Haas experience at Redstone Arsenal.
I was born in Fort Scott, Kan., in 1928, at the outset of the stressed-out 1930s. My father was a civil engineer involved in President Roosevelt's national construction program in Kansas. We frequently moved from one small town to another, necessitating my attending 23 different elementary schools. As a result, I had little opportunity to become lastingly acquainted with fellow students. But I did get to experience life in the "dust bowl."
My extracurricular activities quickly turned to a variety of science-based hobbies, which led me to chemistry—my life's work. This commitment occurred when I was about 13, and it was triggered in part by magazine articles I read. One such article described the role of catalysis in the chemical industry, and another revealed the availability of the Fischer-Tropsch synthetic fuels process in pre-World War II Germany.
In my early chemical self-education, I continually obtained information from local libraries and established a small chemistry lab in my home. I went to high school in Rolla, Mo., but dropped out after three years to obtain some college credits before being drafted. In 1945, I took the entrance exams and started studying chemical engineering at the Missouri School of Mines & Metallurgy, located in Rolla, and now known as the Missouri University of Science & Technology. As things worked out, I was not drafted but had a head start in college.

In the summer of 1947, I transferred to Pomona College, in Claremont, Calif., and became a chemistry major there. This change in academic atmosphere allowed me to carry out undergraduate research with physical organic professor Corwin Hansch until I graduated in 1949. Professor Hansch is now well-known as the originator of quantitative structure-activity relationship (QSAR) studies, which correlate the chemical structure of molecules with their chemical and biological activity by using extended linear free-energy relationships. Believe it or not, at age 90 he is still an active participant in the research enterprise at Pomona.
Advertisement
Professor Hansch and I coauthored my first publication in the Journal of the American Chemical Society during this period (1948, 70, 2495). That little paper described the catalytic synthesis of thianaphthene from ethylbenzene and hydrogen sulfide in the vapor phase. I joined the American Chemical Society at that time, and this year I celebrate being a 60-year member.
I left Pomona and entered graduate studies in chemistry at UCLA in 1949. There I encountered a newly appointed assistant professor who was to become my mentor in organic chemistry and my lifelong friend: Donald J. Cram. He was an organic chemist building a research group to study natural product syntheses and stereochemistry, as well as cutting-edge research problems of interest to physical organic chemists at that time. Cram, Jean-Marie Lehn, and Charles J. Pedersen shared the 1987 Nobel Prize in Chemistry for establishing host-guest chemistry. The senior organic faculty member at UCLA at the time was Saul Winstein, who was avidly pursuing the principles of neighboring-group effects in nucleophilic substitution reactions.
During 1951–53, I held an Atomic Energy Commission Predoctoral Fellowship that had been awarded on the basis of a competitive national exam supported by faculty recommendations. This fellowship freed me from teaching duties and markedly improved my quality of life. I will be forever grateful for this opportunity because I started to eat three times a day and was able to buy a very used car, a large 1938 straight-eight LaSalle sedan. It probably got the same mileage as your typical large RV today. They don't make them like that anymore.
In 1953, the year I obtained a Ph.D. at UCLA, George S. Hammond was an associate professor at Iowa State University, Ames. It was clear that Hammond was on his way to great distinctions in chemistry, and Cram suggested that I postdoc with him.
Once again, I was extraordinarily fortunate, and the 18 months I worked in Ames was outstandingly productive. I learned a large amount of mechanistic chemistry from Hammond and my colleagues in his group. I also witnessed the initiation of mechanistic organic photochemistry as a new research area created by Hammond.
It's amazing that my dream of creating polyhedral borane molecules for use in biomedicine and pharmaceutical applications is now beginning to be realized.
THE FIRST DAY I met Hammond in his office, just after my arrival in Ames, he presented me with a preliminary draft of his now-famous paper "A Correlation of Reaction Rates," which explained his Thermic Postulate (J. Am. Chem. Soc. 1955, 77, 334). This postulate relates transition-state structures of organic chemical reactions to the free energy of the reactants and the products. This was heady stuff because it allowed the proper course of competitive reactions to be predicted in simple terms from either experimentally known or theoretical thermodynamic considerations. Hammond also received the Priestley Medal, in 1976.
During my time in Ames, I was supported by funding from Rohm and Haas for the purpose of elucidating the mechanism of silver-ion-assisted displacement reactions of silver nitroform, AgC(NO2)3, with alkyl halides. This project was unusual in many respects because the C(NO2)3- anion is a weak nucleophile, and its precursors, nitroform and tetranitromethane, are explosive and challenging to handle. Nonetheless, we made progress, and the work was taken up by others in the Hammond group after I left and expanded into a broad study of electrophilic silver ions.
Other items I addressed independently at Iowa were the determination of the nucleophilicity of cyanide ion, the study of the redox chemistry of the tris(p-nitrophenyl)methide ion and its reaction with O2 forming the corresponding hydroperoxide, the search for hydrogen-bond-stabilized transition states among nucleophilic aromatic substitution reactions, and experimental validation of the Thermic Postulate. This was a very engaging postdoctoral experience that resulted in firmly establishing my interest in reaction mechanisms, an interest that has persisted in my polyhedral borane chemistry studies.
Very few academic positions were available in 1954 for physical organic chemists when I was ready to move on from Ames. So my attention was pointed elsewhere by an opening for a senior research chemist at Rohm and Haas's Redstone Arsenal lab—it was officially the Josiah Gorgas Laboratory, named for the chief of the Confederate Army's ordnance corps.
The lab at Redstone Arsenal, which Rohm and Haas operated under a contract with the Army, was small and devoted to exploratory chemistry of energetic materials suitable for solid rocket propellants. At the time, little was known about energetic materials, other than nitro and nitrate compounds. The chemistry section of the lab was divided into organic, physical, analytical, and propellant chemistry groups; later, the organometallic group that I led was added. About 25 Ph.D.s were employed in the chemistry section, along with 30 laboratory assistants.
As was typical of the day, we enjoyed considerable freedom in our research projects. Although there were important short- and long-range problems that we addressed, part of our charter with the Army allowed us generous amounts of time to explore chemistry of our choosing. Publication of our research was encouraged, and the practice became common, although when the lab was first created, all of our work was officially classified. The laboratory was very well equipped. At the time, NMR was just being introduced, and we acquired the earliest Varian instruments.
This unusual research setting led to some spectacular new chemistry by some outstanding chemists. For example, William D. Emmons, who was head of the organic group, was quite a prolific scientist. Among his first contributions to the program was the discovery and application of peroxytrifluoroacetic acid (CF3CO3H) to organic synthesis. This was followed by the Wadsworth-Emmons alkene synthesis. Emmons also made other outstanding discoveries such as oxaziranes, which are three-membered CNO rings. Emmons later led a large portion of the Rohm and Haas research effort at the corporate labs in Spring House, Pa.
Another example is Warren D. Niederhauser. In 1956, he transferred to our lab from the corporate lab in Bridesburg, Pa., to head the chemistry section. Niederhauser generated new concepts for energetic materials, as exemplified by the discovery and development of routes to synthesize organic compounds carrying one or more NF2 substituent groups. The weak NF bonds imparted exceptional performance as oxidant components in solid rocket propellants, and they are important components to some of today's weapons. This NF program was the first attack on organic NF chemistry and, besides the desired explosive organic species RNF2, led to new discoveries that included synthetic routes to the useful precursors NF3, HNF2, and N2F4. Niederhauser later moved to Spring House, where he worked in Rohm and Haas research management. Many people may remember him as the 1984 president of ACS.
Niederhauser is the one who approached me in 1956 with the proposal that I organize and lead the organometallic group to explore borane derivatives as solid rocket fuels. I accepted the offer, learned what borane chemistry there was to be known at the time from the literature, and in due course made this area of chemistry my life's work. This was all the result of chance and being in the right place at the right time. The Rohm and Haas Redstone lab was first organized in 1949 and disbanded in 1974. I am honored by the opportunity I had to participate in its success.
IN 1961, I moved from the Redstone lab to head a Rohm and Haas lab in Bridesburg. This position involved directing research in polymer chemistry and other related projects. Much to my surprise, after about a year there, I was recruited for the position of full professor of chemistry at UC Riverside. This was a new position in organic chemistry, and it was created to augment the university's emerging chemistry graduate program. I jumped at the opportunity and moved to Riverside in January 1962, beginning my academic career.
I did well raising research funding from federal sources, and my research group prospered. Research in polyhedral borane chemistry was pursued with vigor, and many new discoveries arose, such as the unique B20H182- ion, which is the product of B10H102- oxidation.
Most significant was my successful prediction of the existence of metallacarboranes and methods for their synthesis. This discovery connected the fields of borane chemistry and transition-metal chemistry. The concept of highly stabilized transition-metal derivatives of polyhedral carboranes and polyhedral borane anions became popular and spawned a rich array of unique applications, ranging from homogeneous catalysts to extraordinarily stable radioimaging agents.
Our initial discoveries were serendipitous and stand as tributes to very careful synthesis, separation, and characterization studies.
UC Riverside also served as host for my appointment as associate editor of the new ACS journal—at least still new in 1966—Inorganic Chemistry . Edward King served as the editor of the journal, which was published six times a year. Suffice it to say, I enjoyed journal work, dealing with chemical issues, and the mix of many interesting personalities. After King, I became editor-in-chief of Inorganic Chemistry and kept the appointment for 32 years.
In 1968, UCLA expressed interest in my work and contacted me to propose that I join Herb Kaesz in starting an inorganic chemistry division. I accepted the offer and moved my research group to UCLA. It was when I moved to UCLA that I became editor-in-chief of Inorganic Chemistry, and Herb served as my very loyal associate editor for most of my tenure at the journal. I am very proud of Inorganic Chemistry, and I believe the University of Rochester's Richard Eisenberg is doing a splendid job as my successor editor.
UCLA was very good for me. During these years, I vigorously explored many aspects of polyhedral borane, carborane, and metallaborane chemistry, as well as identified potential applications.
Advertisement
One area we explored related to the stability of metallacarboranes in bioactive environments; this suggested the study of radiometallacarboranes containing radioactive transition-metal isotopes for attachment to cancer-cell-selective molecules for the purposes of radioimaging and radiotherapy. This was successfully demonstrated using an essentially indestructible radiocobalt derivative known as a "Venus flytrap" complex.
Another venue we explored was the possible role of late-transition-metal metallacarboranes in homogeneous catalysis. In this area, rhodium complexes proved to be effective catalysts for hydrogenation of alkenes, alkene isomerization, and other reactions under very mild conditions. The mechanisms of these reactions were elucidated, and previously unknown pathways were confirmed.
The scope of metallacarborane structures was also broadened to include species that contained aluminum and gallium as vertices. This was a major expansion of the structural scope and utility of metallacarborane chemistry.
In 1998, I became a University Professor, the highest academic rank in the University of California System, by action of the Board of Regents. It would be easy to say I had it all. But there was one thing still missing: the ability to test the efficacy of carboranes and boranes as therapeutic reagents. For that reason, I "retired" from UCLA in 2006, and the next day, I started my "sunshine career" at the University of Missouri.
Now, we have a new research building for our institute, which was dedicated last fall. It is located across the street from the research reactor and houses a low-energy neutron beam line dedicated to our exploratory BNCT research. These facilities make our institute unique at a time when BNCT is searching for a future.
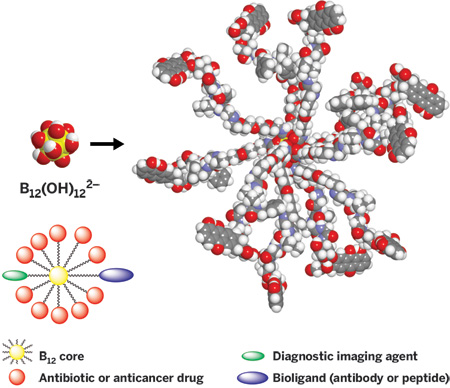
The neutron source is ideally suited for evaluating tumor-cell-selective boron reagents for BNCT. Other objectives of I2NM2 include 12-fold functional derivatives of B12(OH)122-. The BOH vertices of these compounds are easily converted to esters, ethers, and carbamates for linking to a wide variety of payload molecules for the targeted drug delivery of many copies of a pharmaceutical or high-performance MRI contrast reagent. Targeting these large species to selected biological sites is a current challenge.
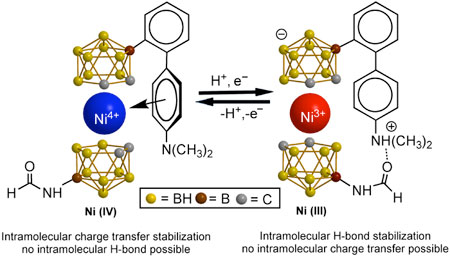
MOST RECENTLY, at the University of Missouri, we have become interested in the use of the basic metallacarborane structure as the motor for a nanomachine. These tiny devices furnish reversible rotation upon oxidation and complementary reduction of the transition-metal center. Such research is currently making good progress, and if successful, many fascinating applications are feasible.
The I2NM2 research program is the culmination of my research carried out during the past 50-plus years. It's amazing that my dream of creating polyhedral borane molecules for use in biomedicine and pharmaceutical applications is now beginning to be realized. This is truly chemistry for the future in which borane structures could become nearly as commonplace as organic structures. I am delighted and continue to be driven by these possibilities and the opportunities afforded me by the University of Missouri.
In conclusion, I wish to thank my excellent teachers over the years, my many former postdoctoral associates, and the large group of students who worked with me in the past. I also appreciate the golden opportunity given to me by Rohm and Haas. I hold my many academic colleagues in high regard for their interactions over the years, as well as the support of all my friends acquired in ACS's Publications Division and the unwavering support given to me while I was editor of Inorganic Chemistry.
Lastly, I thank my wife, Diana, and my family for their loving care and support. Thank you and good day.
Priestley Medal Prologue
Recent medical events in the life of the 2009 Priestley Medalist, M. Frederick Hawthorne, have made it necessary to depart from the usual format of the Priestley Medal lecture. Hawthorne, who is founding director of the International Institute of Nano & Molecular Medicine (I2NM2) at the University of Missouri, Columbia, is recovering from the eradication of tongue cancer and the removal of his thyroid gland, all carried out within the past six months. His prognosis is excellent, but the procedures have left him without his usual strong voice. Hawthorne prerecorded his Priestley lecture during a recovery period between surgeries, and the video will be presented on March 24 at the awards ceremony of the American Chemical Society national meeting in Salt Lake City.
One of the foremost cancer targets for boron neutron capture therapy (BNCT), which has been important for Hawthorne's boron research efforts over the past 50 years, is squamous cell carcinoma of the oral cavity. The majority of BNCT has been supported by the Department of Energy and the National Institutes of Health, and a cadre of neurosurgeons, nuclear engineers, and radiation oncologists have concentrated their BNCT research on the incurable brain tumor glioblastoma multiforme using only three selective boron compounds. More prevalent and tractable malignancies such as cancers of the oral cavity have been controlled with repeated X-ray therapy sessions administered over a long period. BNCT treatment of these same tumor types, once proven, should require only one therapeutic session using a highly selective boron compound to target the tumor cells. Hawthorne has dedicated much of his boron research to synthesizing such reagents in order to reach that end goal.
But due to a lack of success with the virtually incurable glioblastoma multiforme, federal support of BNCT research and development has been dormant for several years, and cancers in patients such as Hawthorne himself have never been subjected to organized clinical trials of BNCT with optimized cell-targeting boron compounds. Although X-ray treatment can be effective for oral and other types of common cancers, depending on the circumstances, Hawthorne believes BNCT offers much more promise and increased cost-effectiveness.
Hawthorne is hopeful that increased funding of NIH by the Obama Administration will put BNCT high on the list of cancer therapies poised for immediate support in strategically organized laboratories such as I2NM2, coordinated with its neutron source partner at the adjoining University of Missouri Nuclear Research Reactor, which houses a new thermal neutron beam built specifically for BNCT research. The coincidence of Hawthorne's experience with cancer and his research focus on BNCT makes the coupling of I2NM2 and the Priestley Medal lecture particularly meaningful, he emphasizes.
Hawthorne wishes to acknowledge the skill and devotion of his physicians Gregory J. Renner (otolaryngology-head and neck surgery), Steven Westgate (oncology-radiation), and Donald Doll (medical oncology), all of Ellis Fischel Cancer Center, and Charles Chapman (internal medicine) at Boone Hospital Center.
Hawthorne regrets that he cannot attend and greet his guests at the awards dinner at the ACS meeting in Salt Lake City.
- 85th Anniversary of the Priestley Medal
- C&EN celebrates the American Chemical Society's highest honor
- Priestley's Medals
- The medals of the minister-scientist who discovered oxygen attest to his fame and infamy
- The Priestley Medalists, 1923-2008
- View a complete list of award recipients
- Living History
- These 12 Priestley Medal winners reflect on winning ACS's most coveted award







Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter