Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Directing Fluorination Differently
After fluorination, directing group can be transformed to make a range of F-containing molecules
by Carmen Drahl
June 1, 2009
| A version of this story appeared in
Volume 87, Issue 22
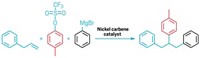
With the help of a versatile nitrogen-containing moiety, chemists have developed a new reaction for adding fluorine atoms to an aromatic ring. Fluorine atoms abound in pharmaceutical compounds because their love of electrons can elicit molecular effects in the body that can be medically therapeutic. Depending on the desired structure, however, it isn't always easy to add fluorine where it's needed, so chemists are continually seeking new fluorination reactions. Now, Xisheng Wang, Tian-Sheng Mei, and Jin-Quan Yu of Scripps Research Institute have developed a palladium(II)-catalyzed reaction that attaches fluorine ortho to a triflamide directing group on benzylamines (J. Am. Chem. Soc., DOI: 10.1021/ja901352k). Other researchers have used pyridine as a directing group in similar fluorinations, but unlike pyridine, the triflamide can subsequently be converted into other functional groups. The new method can therefore open a pathway to a broad range of fluorinated molecules. The reaction's mechanism isn't yet clear, and the process tends to add an extra fluorine atom to certain substrates. The team is working to refine the selectivity and to extend the reaction to more substrates, Yu says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter