Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Three Arsenic Atoms, Three Oxidation States
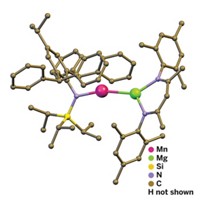
A rare trinuclear arsenic compound stands out because the arsenic atoms are in three different formal oxidation states: As2+, As0, and As1+.
by Stephen K. Ritter
January 19, 2009
| A version of this story appeared in
Volume 87, Issue 3
Compounds with three arsenic atoms are rare to begin with, but Andrey V. Protchenko, Michael F. Lappert, and coworkers of the University of Sussex, in England, have made one that stands out among this small club (Chem. Commun. 2009, 428). “What is particularly interesting about this compound is the three different formal oxidation states of the arsenic atoms all in the same unit,” says chemistry professor Bryan Eichhorn of the University of Maryland, whose group has synthesized other types of catenated arsenic compounds. Lappert’s group made the novel compound by first reacting AsI3 with potassium β-diiminate; the diiminate ligand (L) is quite bulky: RNCHC(Ph)CHNR, where R is 2,6-diisopropylphenyl and Ph is phenyl. The researchers isolated a crystalline intermediate product, AsI2L, and then reduced it by reaction with potassium graphite, KC8, to produce the trinuclear arsenic compound L2As-As=AsL. On the basis of crystal structure data, Lappert and coworkers conclude that there is one As=As bond and that the formal oxidation states for the arsenic atoms under these conditions are As2+, As0, and As1+, from left to right, as depicted.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter