Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Programming Polymers With Solvent And Heat
Stimuli-responsive polymer applications could be useful for chromatography and biosensing
by Jyllian N. Kemsley
August 31, 2009
| A version of this story appeared in
Volume 87, Issue 35
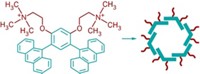
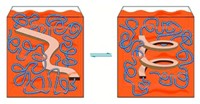
A polymer that changes its characteristics depending on whether it’s heated in a polar or nonpolar solvent can retain the programmed properties when it’s cooled and the solvent is removed, reports a group led by Ken D. Shimizu at the University of South Carolina (J. Am. Chem. Soc., DOI: 10.1021/ja904234w). The ability to use stimuli such as heat to control and manipulate how a polymer recognizes guest molecules is important for applications such as chromatography and biosensing. The solvent-programmable polymer contains an arene ring with carboxylic acid and methyl substituents. When the polymer is heated, the ring can freely rotate around a Caryl–Nimide bond that tethers it to the polymer backbone. In a polar solvent, the ring orients so that the carboxylic acid is exposed to solvent. After the solution is cooled and the solvent is removed, the polymer strongly binds to a guest molecule, ethyl adenine-9-acetate, which is known to form H-bonding interactions with carboxylic acids. In contrast, when the polymer is heated in a nonpolar solvent, the carboxylic acid group becomes less accessible and when cooled only weakly binds to the guest molecule. The solvent-induced switching is reversible and repeatable.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter