Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Screening Toys
Stricter regulatory limits for lead and phthalates create analytical challenges
by Britt E. Erickson
August 31, 2009
| A version of this story appeared in
Volume 87, Issue 35

With the passage of the Consumer Product Safety Improvement Act (CPSIA) of 2008, lawmakers set lower limits for a number of chemicals in children’s products, but they failed to ensure that accurate analytical methods were available to evaluate those products, according to Eileen M. Nottoli, a chemist and environmental attorney with the California-based law firm Allen Matkins. Nottoli was speaking at a symposium sponsored by the Division of Chemistry & the Law at the ACS national meeting in Washington, D.C., earlier this month.
Lawsuits alleging high levels of lead in children’s products have been cropping up in California for quite some time, Nottoli said. In some cases, the claims have been based on a single reading from a screening device. She said she has also seen lawsuits involving lead and cadmium in decorated glassware, and phthalates in pacifiers and other children’s products. In all of those cases, analytical methods for testing the chemicals in consumer products are lacking, she noted.
The rest of the country is now facing a similar situation for lead in consumer products targeted at children and for phthalates. CPSIA gradually sets stricter limits on lead in toys over three years, and it bans six phthalates in kids’ products (C&EN, Feb. 9, page 28). The job of enforcing this law falls on the Consumer Product Safety Commission (CPSC).
Unlike the Environmental Protection Agency and the Food & Drug Administration, which have developed reliable, rigorous, and peer-reviewed methods for quantifying levels of contaminants in food, water, and soil, CPSC has not done the same for the variety of matrixes found in consumer products. “CPSC has published some methods, but peer review appears to be absent,” Nottoli noted.
Of particular concern to Nottoli are CPSC’s methods for quantifying low levels of phthalates and lead in consumer products. The phthalates method relies on gas chromatography (GC) and assumes that you have pure materials, she said. But most commercial phthalates are mixtures, making quantitative analysis difficult because the GC peaks overlap.
The CPSC method for lead paint involves acid digestion followed by inductively coupled plasma (ICP) spectroscopy. The problem with that method is that care must be taken not to remove any of the substrate onto which the paint was applied when collecting the sample. If the substrate contains lead, contamination will inevitably occur, Nottoli pointed out.
She called both methods “insufficient” and emphasized the need for standard reference materials so that labs can validate the methods.
Chemicals in consumer products are regulated under various state and federal laws, many of which are inconsistent with each other. In the case of metals, there’s CPSIA, which lowered the limit for lead in consumer products intended for children from 600 ppm to 300 ppm (effective on Aug. 14), and eventually will lower it to 100 ppm (effective on Aug. 14, 2011). Lead in paint used on such products, however, already is restricted to no more than 90 ppm under CPSIA.
Various states have their own independent regulations that stipulate how much lead can be present in jewelry. California has Proposition 65, which sets daily exposure limits for certain chemicals deemed to be carcinogenic or have reproductive effects.
Under Prop 65, there is no agreement on how to calculate exposure, Nottoli pointed out. “The latest lawsuits are all about potential exposure from hand to mouth,” she said.
For quick screening of lead and other elements, handheld X-ray fluorescence (XRF) analyzers are being widely used by a number of plaintiffs and government agencies, as well as industry, Nottoli said. “It’s a fascinating instrument that offers so much promise, but it is important that we know what its limitations are.”
When improperly used, a handheld XRF device, which looks like a bar-code reader and takes just seconds to minutes to do its job, can yield erroneous results that might fuel unnecessary lawsuits and product recalls. “The potential for recalls can be on the order of tens of thousands of dollars or even over a hundred thousand dollars from lost inventory, shipping, and disposal costs,” Nottoli noted.
But no other method has the advantages of XRF in terms of speed and portability, which are essential for screening hundreds of thousands of toys and children’s products for a quick “go/no go” answer, said symposium presenter Stanislaw Piorek, principal research scientist at Thermo Fisher Scientific NITON Analyzers, a manufacturer of handheld XRF analyzers. In addition, XRF is truly nondestructive and is less expensive than other methods, according to Piorek.
Handheld XRF has been approved by various government agencies for detecting lead in soil, gasoline, and paint, but CPSC has yet to officially approve the technique for detecting lead in children’s consumer products.
Lead can be found in just about any product, Piorek noted, from the button on a garment to the paint on the eye of a rubber duck. With XRF, high levels of lead (on the order of hundreds of thousands of ppm) have even been found in school supplies such as three-ring binders, the soft grip on a pen, and a stapler, he warned. “These are not small numbers in view of the new regulation.”
The downside to XRF is that like all analytical methods it won’t accurately measure lead in products that are heterogeneous without sample preparation. Piorek recommended taking multiple measurements on different sides in several different places to learn more about a particular material and to determine whether sample preparation is needed. XRF also cannot differentiate between lead in paint and lead in a substrate, such as a metal charm.
XRF is intended as a quick screening method—one that complements more accurate but time-consuming methods such as atomic absorption spectroscopy and ICP. It is not intended to give an exact concentration of lead in a sample, Piorek said, but rather allows one to quickly distinguish between products that are compliant and those that may not be. “We need to embrace the concept of screening” and not be obsessed with getting the exact level of a particular chemical in a product.
Detection of phthalates, albeit indirectly, may also be possible with XRF, noted symposium presenter Richard A. Kagel, lab director of California-based K Prime, an analytical chemistry testing and consulting firm. By using XRF to measure chlorine, you can identify whether the material contains polyvinyl chloride (PVC). Phthalates are added to PVC plastic to soften it.
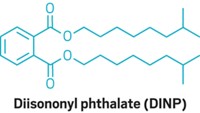
Phthalates, like lead, present their own set of analytical challenges. CPSIA bans six phthalates—di(2-ethylhexyl) phthalate, dibutyl phthalate, benzyl butyl phthalate, diisononyl phthalate, diisodecyl phthalate, and di-n-octyl phthalate—in products intended for children 12 years and under. However, because phthalates are so ubiquitous, each of the banned chemicals can be present at concentrations of up to 1,000 ppm.
Phthalates are found in a wide variety of consumer products, including vinyl toys, raincoats, and lunchboxes; cosmetics such as nail polish and lipstick; perfume and air fresheners; personal care products like deodorants, shampoo, and lotion; paints; and pharmaceutical coatings. In fact, the plastic badges worn by all of the conferees at the ACS national meeting are about 40% by weight phthalates, Kagel pointed out. Phthalates are also found in many common laboratory materials, which makes their analysis quite challenging, Nottoli said.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter