Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
GFP In Motion
Photochemistry: Ultrafast method reveals how green fluorescent protein changes during proton transfer
by Celia Henry Arnaud
November 16, 2009
| A version of this story appeared in
Volume 87, Issue 46

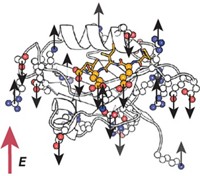
Chemists have used ultrafast Raman spectroscopy to map the structural evolution of the green fluorescent protein (GFP) chromophore during proton transfer. The findings not only help explain the workings of GFP but could also lead to a better fundamental understanding of proton transfer, which drives key biological processes.
“Since the discovery in our lab in 1995 that the GFP chromophore exists in two states connected by ultrafast excited-state proton transfer, it has been of enormous interest to understand the motions responsible for converting from one state to the other during this reaction,” says Stanford University chemistry professor Steven G. Boxer, who was not involved with this research.
Richard A. Mathies and coworkers of the University of California, Berkeley, have now accomplished this by using femtosecond stimulated Raman spectroscopy to capture structural snapshots of the transition (Nature 2009, 462, 200). In this method, one laser initiates a photochemical process. Then, pulses from a picosecond Raman pump laser and a 20-femtosecond probe laser interrogate the system. This stimulates Raman signals that provide snapshots of the molecular structure every 25 femtoseconds.
“This is the first molecule where we’ve gotten an opportunity to see the structural evolution of the excited state after the photon goes in but before the chemical reaction occurs,” Mathies says.
Although many motions are involved, the wagging of a phenoxy group in the chromophore dominates in aligning it for proton transfer. “As the phenoxy group moves to line everything up, all the electrons shift” across the chromophore, Mathies says.
“It is amazing to see, in real time and bond by bond, how the chromophore changes during this excited-state proton-transfer reaction,” Boxer says.
“Proton transfer is an all-important process in biology, a key process in bioenergetics, enzymology, signal transduction,” and more, says John T. M. Kennis, a biophysicist at the Free University of Amsterdam. “If we aspire to construct an artificial solar cell, we will need absolute control not only of electron transfer but also of proton transfer.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter