Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Skeleton Key May Defuse Flu
Antibodies bind a flu protein nook common to many viral strains
by Carmen Drahl
March 2, 2009
| A version of this story appeared in
Volume 87, Issue 9
A FAMILY OF ANTIBODIES neutralizes multiple types of flu by targeting a weak spot in the virus, according to a pair of studies. The discovery could point the way to treatments that shield people from both seasonal and pandemic flu.

Every new year brings a new flu season and a new flu vaccine to go with it. Flu vaccines are developed by predicting which types of flu will dominate the coming season, structural biologist Ian A. Wilson of the Scripps Research Institute says. "If anything different comes along, the vaccine won't be as effective," he says.
Most vaccines elicit antibodies to hemagglutinin, a protein on the virus's surface. But the region of hemagglutinin that the antibodies target mutates rapidly, allowing viruses to elude human immune systems. Now, independent teams led by Wilson and Wayne A. Marasco of the Dana-Farber Cancer Institute, in Boston, have found antibodies targeting a portion of hemagglutinin that is less variable and is consistent in many types of flu.
Each team started with a library containing large numbers of antibodies compiled from human volunteers. Marasco's library was built in-house, whereas Wilson collaborated with Dutch biotechnology company Crucell. They fished out antibodies of interest by using avian flu hemagglutinin as their lure. With Ruben O. Donis of the Centers for Disease Control & Prevention, Marasco found that his handful of hits suppressed disparate types of flu in mice, a finding replicated by Wilson's team.
The antibodies are versatile because they don't interact with hemagglutinin's variable region. Two other groups found similar antibodies last year, but they didn't unambiguously describe their mechanism of action.
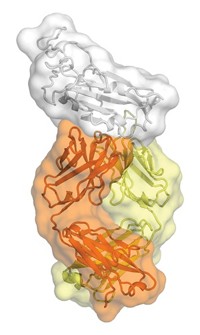
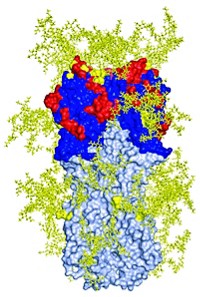
Now, to get at the mechanism, Marasco and Dana-Farber colleague Jianhua Sui teamed with Robert C. Liddington and William C. Hwang of the Burnham Institute for Medical Research, in La Jolla, Calif., to obtain a crystal structure of an antibody bound to avian flu hemagglutinin (Nat. Struct. Mol. Biol., DOI: 10.1038/nsmb.1566).
Independently, Wilson and Damian C. Ekiert obtained that structure, as well as one of an antibody bound to hemagglutinin from the 1918 epidemic flu (Science, DOI: 10.1126/science.1171491). In every structure, the antibody targets the same pocket, which is in the stem of hemagglutinin rather than its variable head.
The stem pocket is conserved because it is involved in a conformational change that's crucial for viral infection, Wilson says. With an antibody bound in this pocket, hemagglutinin can no longer change its shape, a step required before the virus can fuse with a cell and send in its genetic material.
Physicians might someday be able to inject this type of antibody into people infected with the flu, or the stem pocket could be used to make a new vaccine that doesn't need retooling every year, Marasco says.
"Hopefully, these works will provide a starting point for rational vaccine design and ultimately improve the therapeutic treatment of influenza," says Zihe Rao, an expert in crystallography of flu proteins at Tsinghua University, in Beijing.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter