Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
MOF Housekeeping Improves
Supercritical CO2 scrubs porous metal-organic frameworks, increasing surface area for applications
by Stephen K. Ritter
January 7, 2009

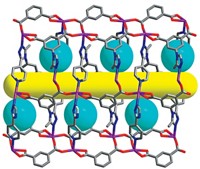
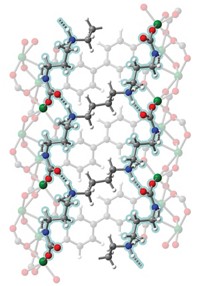
METAL-ORGANIC FRAMEWORKS (MOFs) have just received a big boost in their already superlative ability to adsorb molecules with the advent of a supercritical CO2 processing technique that thoroughly clears the pores of the crystalline materials (J. Am. Chem. Soc., DOI: 10.1021/ja808853q). The development, reported by chemists Andrew P. Nelson, Omar K. Farha, Karen L. Mulfort, and Joseph T. Hupp of Northwestern University, significantly increases the available internal surface area of MOFs compared with standard solvent evaporation techniques currently used to clean newly synthesized materials.

MOFs are metal clusters interconnected by organic linker groups, a design that endows the materials with large pores, open channels, and huge internal surface areas for adsorbing molecules. But experimentally measured surface areas for the frameworks typically fall short of those hinted at by computer models and crystal structures.
This discrepancy is attributed to the collapse of pores between MOF particles, to internal MOF channels that collapse after solvent removal, or to residual solvent or unreacted reagent molecules, Hupp explains. These collapses and the errant molecules block access to some adsorption sites, thereby limiting the materials' full potential for catalysis or for purifying and storing gas molecules such as H2, CO2, and CH4.
Scientists try to work around the problem by "activating" MOFs, a process that includes heating the materials under vacuum to drive off unwanted molecules. However, the procedures yield inconsistent results.
Complete removal of residual molecules from the pores is critical to the commercial use of MOFs, notes UCLA's Omar M. Yaghi, a MOF specialist. Supercritical fluid treatment has been used to activate amorphous polymers and silica-based materials, he notes, but it has rarely been effective with porous crystalline materials.
In the Northwestern team's "supercritical drying" method, the researchers replace the synthesis solvent with more volatile ethanol and then flood the sample with liquid CO2. Then they heat the sample under pressure to reach the CO2 critical point of 31 °C and 73 atm, where CO2 exhibits properties between those of a gas and a liquid.
After 30 minutes under supercritical conditions, the CO2 is vented, sweeping out the detritus and leaving behind cleaner, more intact and accessible pores. The researchers observed up to a 12-fold increase in the internal surface area of the zinc carboxylate MOFs they tested compared with the same MOFs treated by standard activation methods.
MOF internal surface areas can vary greatly depending on the activation process and from one lab to the next, observes Gérard Férey, a MOF specialist located at the University of Versailles' Lavoisier Institute, in France. The supercritical technique appears to work consistently "like a hurricane" to clear the pores in the MOF skeleton and increase the available surface area, he adds. Hupp's method represents "a real standard for MOF activation" that will help avoid the surface-area discrepancies of the past, Férey says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter