Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Graphene Via Arc Discharge
Electrical method yields sheets of carbon a few atoms thick
by Mitch Jacoby
March 3, 2009
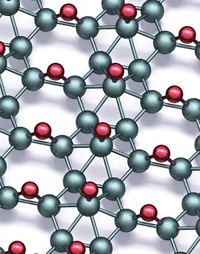
Fitted with graphite electrodes, arc-discharge instruments , which previously have been used to make nanotubes and other helical carbon structures, can be operated in a way that yields graphene (right image).
Fitted with graphite electrodes, arc-discharge instruments , which previously have been used to make nanotubes and other helical carbon structures, can be operated in a way that yields graphene (right image).
A simple electrical method provides an alternative way to prepare graphene samples, according to researchers in India. Graphene—one-atom-thick sheets of carbon packed in a honeycomb structure—has recently attracted considerable attention for its outstanding electronic and mechanical properties (C&EN, March 2, page 14). Single- and few-layer forms of the material have been made by peeling apart graphite, by multistep chemical methods, and by other laborious and complex techniques.
Now, Chintamani N. R. Rao and coworkers at the Jawaharlal Nehru Center for Advanced Scientific Research, in Bangalore, have demonstrated that a simple arc-discharge procedure yields graphene samples that are two to four layers thick (J. Phys. Chem. C, DOI: 10.1021/jp900791y). After maintaining a high-current, high-voltage arc between graphite electrodes in the presence of hydrogen, the team collected graphene flakes from one part of the interior of the apparatus. In contrast, they found multiwalled nanotubes, "onions," and other carbon materials specifically in the vicinity of the cathode.
The group also showed that the technique can be used to dope graphene with boron and nitrogen when the electrical discharge is formed in the presence of diborane and pyridine, respectively. By terminating dangling bonds (unsatisfied valencies) on carbon, hydrogen appears to play a key role in preventing the graphene sheets from rolling into nanotubes and graphitic particles, the team says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter