Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Business
'Beta Testing'
ACS Meeting News: Using chemistry tools to refine an Alzheimer's drug candidate
by Carmen Drahl
April 5, 2010
| A version of this story appeared in
Volume 88, Issue 14
When it comes to treating Alzheimer’s disease, there isn’t one strategy that everyone agrees will work. But blocking a protease enzyme known as ß-site amyloid-cleaving enzyme 1 (BACE or ß-secretase) is a promising option.
At the American Chemical Society national meeting in San Francisco, Andrew W. Stamford, director of medicinal chemistry at Merck & Co. in Kenilworth, N.J., described how he and his colleagues discovered small-molecule inhibitors of BACE. Work toward the inhibitors, the most advanced of which has reached Phase I clinical trials, was begun at Schering-Plough.
Developing a BACE blocker that can be taken orally and can reach the brain efficiently isn’t easy. “You have to build a molecule large enough and hydrophobic enough to bind to the enzyme but small enough to cross the blood-brain barrier and reach the brain,” Stamford told C&EN.
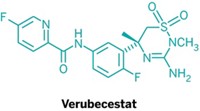
For Stamford’s team, the challenges started early, when conventional screens for BACE blockers yielded no hits. An NMR screen yielded a promising hit: an isothiourea that interacts with the catalytic aspartate residues in the active site of BACE (J. Med. Chem. 2010, 53, 942). But isothioureas are too susceptible to hydrolysis to be viable drug leads. An X-ray crystal structure of an isothiourea bound to BACE helped the team come up with alternatives. They settled on five-membered ring heterocycles called iminohydantoins. The molecules preserved the binding to the active site and were “nicely set up” to explore binding contacts with pockets near the active site as well, Stamford said.
The early compounds were weak BACE inhibitors—too weak to evaluate with conventional assays, according to Stamford. With help from colleagues with expertise in NMR and X-ray crystallography, the team discovered an iminohydantoin with nanomolar binding affinity to BACE. But its properties, including molecular weight and hydrophobicity, were not ideal for an orally active, brain-targeted drug. So the team got rid of a bulky urea-containing substituent. They took a hit in binding affinity, but the resulting compounds had better druglike properties (J. Med. Chem. 2010, 53, 951).
The team then set out to regain the affinity they’d lost while also looking to improve druglike properties. They saw big improvements when they expanded the size of the heterocyclic ring from a five-membered iminohydantoin to a six-membered iminopyrimidone. Adjustments to an aromatic group attached to the iminopyrimidone improved binding contacts with some interconnected pockets near the BACE active site. This first yielded SCH-785532 and later yielded SCH-1359113, two compounds that could be administered orally. The compounds also effectively lowered levels of amyloid-, a peptide associated with Alzheimer’s, in the cerebrospinal fluid and cortex of rodents, which suggests that the compounds reach the brain efficiently. In San Francisco, Stamford also discussed how a more advanced BACE inhibitor lowers levels of amyloid- in monkeys. The structure of that compound was not disclosed.
The take-home lesson from the Merck team’s BACE effort is that chemists “must leverage all the tools they have available” when drug candidates aren’t amenable to conventional assays, Stamford told C&EN. “You can’t underestimate the hard work it takes to make an impact on a challenging target.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter