Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
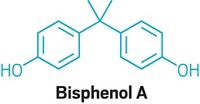
As I was reading Rudy Baum's outstanding editorial "BPA Craziness," I was struck by the similarities between the current craziness over bisphenol A (BPA) and perchlorates (C&EN, March 1, page 5).
Just as BPA is the "best" compound for use in food-container linings, ammonium perchlorate (AP) is the best oxidizer for our many class-1.3 rocket propellants. In fact, those propellants exist only because AP is the oxidant. Alternatives do not perform as well and are more expensive and/or more hazardous.
The use of AP and potassium perchlorate (KP) in both military and civilian pyrotechnics is well established. In fireworks, AP and KP are used because they function well as oxidizers, particularly in colored-star compositions. Alternatives such as chlorates, nitrates, and tetrazoles have disadvantages.
The primary "vulnerable population" concern for BPA use is infants and children. This is also the case for perchlorates. I know the perchlorate anion is preferred over iodide for uptake in human thyrocytes through the sodium/iodide symporter, and iodide inhibition can result in the reduction of thyroid hormone production critical for the normal growth and development of fetuses, infants, and young children. However, does this concern merit calls by environmentalists for the banning of perchlorates in propellants and pyrotechnics? I think not.
I believe all the points Baum makes about BPA can be made about perchlorates. The editorial could easily have been titled "Perchlorate Craziness," and the first paragraph changed to read, "The sad saga of perchlorates in propellants and pyrotechnics reveals much about what is wrong with some environmentalists today."
Roger L. Schneider
Whitefish Bay, Wis.
While I agree that we live in a very chemophobic environment, and any mention of a potential negative health effect of a compound can cause a public uproar calling for its ban, I must disagree with Baum's assessment of BPA not being related to estrogen structurally. Medicinal chemists familiar with estrogens would readily classify the structure as a potential estrogen mimetic. We've been surprised on many occasions with the promiscuity of estrogen receptors.
Diane Hauze
St. Davids, Pa.
In the early 1950s, I worked on process development for bisphenol A (BPA) for Allied Chemical. Later, I did process development for epoxy resins for Reichhold. And in my last position at NASA, I used epoxy resins to make fire- and laser-resistant transparent "windows."
I am now 91 years old and have no apparent health problems from having worked with BPA. It is such a useful substance that it would be a shame to try to prohibit its use. Some people are trying hard to do that, and I see that Congress has just allocated more millions of dollars to study it.
One argument that is being used against BPA use is the fact that bacteria do not grow as well in petri dishes made of polycarbonate as they do in glass. That really should be an argument in favor of its use in things like water bottles and as epoxy-based coating in cans because BPA's bacteriostatic and fungistatic properties help the contents of those bottles and cans stay consumable longer.
Now we are seeing aluminum and stainless steel water bottles. I wonder what happened to the rumors that Alzheimer's disease was related to aluminum and that nickel and chromium were toxic.
Neither is glass innocuous. I once was employed by a company that supplied hospital solutions in glass bottles. One of the tests we conducted was to keep some of the bottled solutions at elevated (desert) temperature. After several months, glass separated in layers and broke up into flakes and particles of various sizes.
There are so many other phenolic materials that people use daily. Phenolic compounds are in mouthwash as well as in many spices and herbs. Joseph Lister revolutionized surgery by sterilizing instruments and wounds with 3 to 5% phenol solutions. Yet some in Congress want to ban all phenolic substances from commercial use. If BPA is banned, then thyme, cloves, vanilla, and many other spices and herbs, even red wine (resveratrol), should also be banned and subject to the same rigorous tests that are being conducted on BPA.
George Fohlen
Sonoma, Calif.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter