Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Lithium Cation Isolated Within Fullerene
Stabilized crystal structure could find use in electronics applications
by David Pittman
June 28, 2010
| A version of this story appeared in
Volume 88, Issue 26
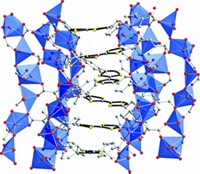
Japanese scientists have for the first time isolated and determined the molecular and crystal structure of a cationic metallofullerene, Li@C60 (Nat. Chem., DOI: 10.1038/nchem.698). Although fullerenes with a metal or other small molecule inside the soccer-ball-like structure are nothing new, the lithium in this fullerene cage is both charged and, notably, off-center. Hiroshi Sawa of Nagoya University and Hiromi Tobita of Tohoku University note the ion state has not been seen before in conventional metallofullerenes. Their compound forms (Li@C60)(SbCl6) crystals (shown) that are 99% pure, a level not reached before, the researchers point out. Metallofullerenes are promising in the field of electronics, but their utility may be limited by their reactivity toward empty fullerenes that coexist in soot and solvent extracts. The crystalline structure prepared by the researchers stabilizes the electrostatic force between the Li+ cations and the SbCl6 – anions, preventing unwanted reactions with empty fullerenes. “Such position control by an external field can be widely used in electronics applications, including the single molecular switch and ferroelectric sheets,” Sawa writes.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter