Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Versatile Catalyst Delivers CF3 Groups
Palladium-mediated method adds influential moiety to a variety of molecules
by Carmen Drahl
June 28, 2010
| A version of this story appeared in
Volume 88, Issue 26
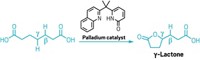
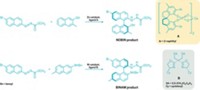
Tack one more reaction onto the list that bulky ligands from MIT help accomplish—adding trifluoromethyl groups to aromatic rings. Trifluoromethyls make molecules more lipophilic and metabolically stable, among other desirable properties, so chemists in the drug and agrochemical industries are constantly trying to prepare them more efficiently. Stephen L. Buchwald and colleagues at MIT previously used a bulky, phosphine-containing ligand to install fluorine atoms on aromatic rings (C&EN, Aug. 17, 2009, page 7). Now, they’ve applied a related ligand to trifluoromethylations, with help from a catalytic amount of palladium and a silicon reagent that acts as a trifluoromethyl source (Science 2010, 328, 1679). So far, the reaction (shown) works best at elevated temperatures. But it doesn’t require directing groups, and it works with a wide range of aryl chlorides, including heteroaromatic chlorides. The work is “one of the most important breakthroughs in fluoroalkylation chemistry since the late 1960s,” says David A. Vicic, an organometallic chemist at the University of Hawaii, Manoa. “The future of this chemistry will be to get the reactions to go using cheaper reagents,” he adds.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter