Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Jack-Of-All-Trades Detergents
Biochemistry: Versatile molecules aid multiple stages of membrane-protein structure determination
by Carmen Drahl
November 8, 2010
| A version of this story appeared in
Volume 88, Issue 45
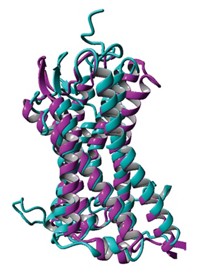
A new class of detergents with quaternary carbon centers could become handy tools for obtaining structures of membrane proteins, a new study suggests. Membrane-embedded proteins play crucial roles in biology and are often drug targets, but it's notoriously difficult to determine what they look like.
Detergents, amphiphilic molecules with polar heads and nonpolar tails, play several roles in membrane protein structure determination. They extricate proteins from their native environment in a cell membrane, keep the freed proteins stable, and aid crystallization. But conventional detergents don't always do all those jobs well.
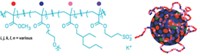
From a molecular standpoint, detergents tend to be either very flexible or very rigid. To come up with new tools, "we wondered if we could tune that flexibility," says chemist Samuel H. Gellman of the University of Wisconsin, Madison.
Gellman's postdoctoral colleague Pil Seok Chae had the idea to create detergents with intermediate flexibility by fusing various amphiphiles. So far, the most versatile family of detergents are made of two molecules of dodecyl maltose, a workhorse amphiphile in crystallography. When fused at one carbon, the two molecules resemble scissors with polar carbohydrate handles and nonpolar alkyl blades.
Through a host of collaborators including Brian K. Kobilka and Soren G. F. Rasmussen of Stanford University, the Wisconsin team tested its new detergents on different membrane proteins. The molecules both stabilized the proteins and helped crystallize them (Nat. Methods, DOI: 10.1038/nmeth.1526). In unpublished work, Kobilka's team has used one of Gellman's detergents to help obtain structures of two different forms of a membrane-spanning G-protein-coupled receptor. Gellman's and Kobilka's teams together have applied for a patent on the new amphiphiles.
"It's very difficult for chemists to do a project like this," Kobilka says. "They have to be creative, and they have to harass us membrane protein biochemists to try their reagents and be critical about the data we give them."
The new detergents are exciting because they seem to stabilize a wide range of proteins and are useful for many aspects of membrane protein work, from purification to crystallization, UCLA biochemist James U. Bowie says. "I certainly look forward to trying them."




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter