Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Speedier Electron Transfer
By strengthening the interface between two proteins, researchers quicken the pace of long-range electron transfer
by Jyllian N. Kemsley
November 22, 2010
| A version of this story appeared in
Volume 88, Issue 47
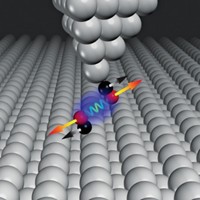
By altering the interface between myoglobin (Mb) and cytochrome b 5 to strengthen their interaction, researchers have sped up long-range electron transfer between the two proteins by several orders of magnitude (Science, DOI: 10.1126/ science.1197054). Long-range, interprotein electron transfer plays a role in a variety of biological redox processes, including keeping the iron atom in Mb reduced so it can bind to O2 in muscle cells. The research team, led by Brian M. Hoffman and Michael R. Wasielewski at Northwestern University, used Brownian dynamics simulations to determine the optimal Mb residues—two aspartates and a glutamate—to replace with lysines to promote complex formation between Mb and cyt b 5. The researchers then used femtosecond transient absorption spectroscopy to probe the rate of electron transfer in the altered system. They found a distribution of rate constants, on the order of 109 to 1010 s–1, indicating an ensemble of complexes and reactions. Typical interprotein electron transfer rates are 106 s–1, although the ultrafast transfers in photosystems I and II in photosynthesis run at 1011 to 1012 s–1. Further study should improve understanding of the interplay between protein-protein binding and electron transfer, Hoffman says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter