Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Catalytic Reaction Activates Alkanes
by Stephen K. Ritter
December 20, 2010
| A version of this story appeared in
Volume 88, Issue 51
Carbon-hydrogen bond activation took a big step forward in 2000 when John F. Hartwig and coworkers reported an organometallic catalyst capable of directly converting normally unreactive alkanes into compounds with useful functional groups tacked on to the ends.
At the time, alkane functionalization was typically carried out by catalytic photochemistry. Selective, lab-scale C–H bond activation via transition-metal complexes was possible, but those reactions required stoichiometric amounts of expensive reagents. At the industrial scale, processes such as petroleum cracking and free-radical halogenation are used, but they create mixtures of products.
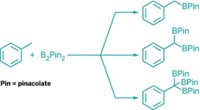
Hartwig's group, then at Yale University, working in collaboration with Thomas C. Semple of Shell Chemicals, discovered a rhodium catalyst in which the metal atom is sandwiched between labile hexamethylbenzene and pentamethylcyclopentadienyl ligands. The researchers used the complex to catalytically couple linear alkanes with commercially available borane reagents to make alkylboranes. The beauty of the borane products, Hartwig says, is that they can be easily converted to alcohols, amines, alkenes, and other derivatives using textbook organic chemistry.
Organic chemists were optimistic that practical applications of industrial-scale catalytic alkane functionalizations were within reach. Indeed, Hartwig's group—now relocated to the University of Illinois, Urbana-Champaign—and others have developed alkane functionalization chemistry that is starting to make its way into pharmaceutical and fine chemicals development.
"We continue to work on the end-functionalized alkanes, including some new unpublished work on the functionalization of alkyl C–H bonds with silicon reagents," Hartwig says. "We have shown that the functionalization of unactivated primary C–H bonds with boron reagents occurs in ethers, amines, and alkyl halides, in addition to alkanes." Hartwig has also collaborated with Marc A. Hillmyer of the University of Minnesota, Twin Cities, to end-functionalize polyolefins to form alcohols that can be further converted to aldehydes and amines for generating new materials.
Perhaps most important right now, Hartwig says, is that the original research has evolved into practical borylation of aromatic C–H bonds using an iridium catalyst. The resulting aryl boronate esters can be used in Suzuki-Miyaura cross-coupling, oxidized to phenols, and converted to aryl halides, aryl amines, aryl ethers, or aromatic nitriles. This chemistry has been used by several research groups, including his own, to synthesize natural products.
"What is needed to make reactions of aliphatic C–H bonds truly practical is the next generation of catalysts that allows us to conduct reactions of saturated compounds with the kind of reactivity we see with arenes," Hartwig says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter