Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Functionalized Nanoparticles For Clinical Diagnostics
by Mitch Jacoby
December 20, 2010
| A version of this story appeared in
Volume 88, Issue 51

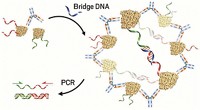
A decade ago, Northwestern University's Chad A. Mirkin and Robert L. Letsinger led a team in devising a nanoparticle-based method for detecting DNA that provided far greater sensitivity than standard fluorometric detection methods at the time. The advance led to the founding of a Northbrook, Ill.-based nanobiotechnology company, Nanosphere, which today makes a line of medical diagnostic systems based on the technology and sold under the Verigene trade name.
COVER STORY
Functionalized Nanoparticles For Clinical Diagnostics
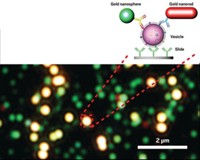
The detection process relies on gold nanoparticles functionalized with DNA or RNA oligonucleotides or with antibodies that selectively bind to complementary nucleic acid or protein targets, respectively. By capturing the nanoparticle-tagged targets on a solid support via hybridization reactions, the targets can be detected with high sensitivity as a result of the intensity with which nanoparticles scatter light. For example, compared with the enzyme-linked immunosorbent assay method, a technique commonly used to detect the presence of an antibody or antigen, the nanoparticle method is at least 100 times as sensitive.
According to Mirkin, Nanosphere has received Food & Drug Administration clearance for five of its diagnostic tests. These include tests for early detection of respiratory illnesses and blood coagulation disorders and a test to gauge a patient's ability to metabolize warfarin, an anticoagulant medication. The company is also developing diagnostics for sensitive detection of markers for cancer and heart disease.
Looking back to the mid-1990s, "we started with the idea of functionalizing nanoparticles with DNA to do programmed materials synthesis," Mirkin says. The strategy, he explains, was to organize lattices using particles as atoms and DNA as bonds. "These structures turned out to have extraordinary and unexpected properties that provide major advantages for medical diagnostics," Mirkin adds. "It's a real success story for chemistry."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter