Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
A New Alternative To FRET
Bioanalysis: Surface energy transfer works over larger distances than does the more-established fluorescence technique
by Rajendrani Mukhopadhyay
November 9, 2010
A team of scientists in Florida and China have found a longer molecular ruler. They have demonstrated that an optical technique called surface energy transfer (SET) can increase the distances that scientists can measure within or between proteins in a live cell (J. Am. Chem. Soc., DOI 10.1021/ja106360v).
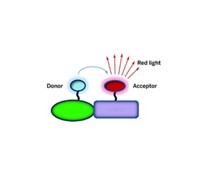
Fluorescence resonance energy transfer (FRET) is the dominant optical technique to measure distances within or between biomolecules. Scientists modify each molecule in a pair with a fluorescent label, or fluorophore, and use light to excite one fluorophore into a higher energy state. That energy then transfers to the other fluorophore, which leads the first to emit less light. The energy transfer's efficiency depends on how far apart the two molecules are from one another and is based on a 1/R6 relationship, where R is the distance. Because of this distance dependence, FRET is only practical for distances less than 10 nm.
FRET's limits had frustrated Yan Chen, a postdoctoral fellow in the laboratory of physical chemist Weihong Tan, at the University of Florida, Gainesville. She and members of Tan's team at Hunan University and Xiamen University were studying a membrane-bound protein called PTK7, which is overexpressed in some forms of leukemia. They had previously discovered that two molecules bound to the protein: an antibody called anti-PTK7 and a short stretch of an oligonucleotide. To pursue PTK7 as a target for cancer therapies, the researchers needed to map the two binding sites in exquisite structural detail. But the sites appeared to be too far apart to measure by FRET. So Chen and her colleagues turned to FRET’s cousin, SET.
Over the past decade, scientists have begun to move SET from the purely theoretical into limited practice. People use it to study RNA folding or DNA-protein interactions in solution. In SET, a metal nanoparticle acts as the energy acceptor, replacing FRET's second fluorophore. The electronic properties of the nanoparticle make the energy transfer more efficient. As a result, energy can travel longer distances between the two molecules than in FRET, with a distance dependence of 1/R4.
The researchers wanted to see if SET could measure distances in a protein structure within a live cell, a new application for the technique. They modified gold nanoparticles with the PTK7-binding oligonucleotides and tagged anti-PTK7 with a fluorescent dye. The investigators then monitored the antibody's fluorescence by flow cytometry as the two molecules bound PTK7. Based on these measurements, they calculated that the distance between the two sites is 13.4 nm. The data provide the team with the first detailed information about where molecules bind on the protein’s surface.
SET could have "great potential" as a tool for mapping out the structures of membrane-bound proteins, Tan says. These proteins must sit in greasy membranes to fold properly, so they are often incompatible with techniques that examine water-soluble proteins, such as X-ray crystallography and nuclear magnetic resonance. Those methods also demand skill and funds, meanwhile SET "is a relatively easy technique," says Tan.
Molecular biophysicist Geoffrey Strouse at Florida State University says the work in live cells is an important advance that uncovers the possibility of studying dynamic events, such as the opening of pores in cell membranes.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter