Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Fluoride Sensor Also Fluorinates
A sulfonium borane complex that traps fluoride ions can turn around and use them in substitution reactions
by Stephen K. Ritter
March 7, 2011
| A version of this story appeared in
Volume 89, Issue 10
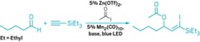
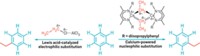
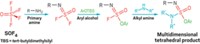
A sulfonium borane complex that selectively traps fluoride ions under aqueous conditions can turn around and hand off the fluoride in nucleophilic substitution reactions under anhydrous conditions, a feat that should open new possibilities in fluorine chemistry, according to Haiyan Zhao and François P. Gabbaï of Texas A&M University (Org. Lett., DOI: 10.1021/ol200129q). Gabbaï’s group previously prepared a sulfonium borane salt in which dimethylsulfonium and arylborane groups on adjacent rings of naphthalene work together to trap a fluoride ion and function as a fluoride sensor. The researchers figured out that they can demethylate the sulfur using a phenylthiolate salt, untethering the fluoride to form an anionic fluoroborate that is an efficient room-temperature nucleophilic fluorinating reagent. This new reagent works on a variety of substrates, including alkyl halides and aromatic compounds bearing nitro (shown), sulfonyl chloride, and other electron-rich substituents. “The benefit of our approach is that we can start from aqueous fluoride solutions and after one simple capture step carry out fluorination reactions in dry organic solvents,” Gabbaï says. This capability might benefit 18F radiolabeling of organic reagents used in positron emission tomography, he suggests, because the short-lived 18F– is first prepared in aqueous solution and requires fast processing.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter