Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Amphotericin B Mystery Solved
Decades-long question about Antifungal Agent’s Mechanism is answered
by Stu Borman
March 21, 2011
| A version of this story appeared in
Volume 89, Issue 12
Chemical synthesis has resolved the long-standing uncertainty about how the potent but highly toxic antifungal natural product amphotericin B works. The findings could lead to rational design of analogs with fewer side effects than the natural compound.
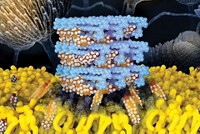
Amphotericin B, a cyclic polyene macrolide, is used to treat progressive and life-threatening fungal infections, but it has serious side effects, including potentially fatal cardiac or cardiopulmonary arrest. Scientists have known that its activity is due to formation of ion channels, but despite decades of research, they have not been able to determine how the channels assemble.
Three hypotheses to explain how amphotericin B molecules gang up to create cell-killing ion channels in fungal cell membranes differ in the molecular interactions that drive ion-channel formation. In one model, mycosamine and carboxylate groups on neighboring amphotericin B molecules form noncovalent bonds. In the second model, mycosamine and/or carboxylate groups form polar interactions with head groups of membrane phospholipids. In the third, the mycosamine and/or carboxylate groups bind to membrane-embedded sterols such as ergosterol and cholesterol.
This last model is the correct one, Martin D. Burke and coworkers at the University of Illinois, Urbana-Champaign, report in a recent paper (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.1015023108). Burke’s team came to this conclusion by synthesizing and testing derivatives of amphotericin B that lack the mycosamine group, the carboxylate group, or both. They also found that amphotericin B requires the mycosamine group—but not the carboxylate—for its molecules to interact with ergosterol or cholesterol and assemble into ion channels.
Maciej Baginski, an expert on the mechanism of action of antifungal polyene macrolide antibiotics at Gdansk University of Technology, in Poland, comments that the finding “that amphotericin B molecules without the carboxyl group can still form channels and exhibit antifungal activity is a very new and rather surprising observation from the perspective of all 50 years of studies on the molecular mechanism of amphotericin B action.”
The findings “will help focus efforts toward the rational optimization of the therapeutic index of this clinically vital but also highly toxic antimycotic,” Burke and coworkers note in their paper. “These studies vividly demonstrate the power of synthesis-enabled functional group deletions to illuminate highly elusive fundamental underpinnings of small-molecule function.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter