Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Rigging Cross-Metathesis
Organic Synthesis: Molybdenum catalyst generates less stable Z olefin
by Bethany Halford
March 28, 2011
| A version of this story appeared in
Volume 89, Issue 13
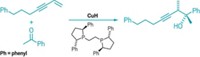
Scientists who’ve lost sleep over the metathesis reaction’s preference for forming E olefins can finally catch some Zs—Z olefins, that is. Using specially designed molybdenum catalysts, chemists have figured out a way to rig cross-metathesis reactions to produce less stable Z double bonds (Nature, DOI: 10.1038/nature09957).
“Alkenes are starting materials for an impressive number of some of the most important reactions in chemistry,” says Amir H. Hoveyda, the Boston College chemistry professor who spearheaded the research in collaboration with MIT chemistry professor Richard R. Schrock. “The stereochemistry of a starting alkene is often critical to the stereochemical outcome of its functionalization”—something Hoveyda’s group demonstrates by using the new reaction en route to the immunostimulant KRN7000. The reaction can also be used to make natural products containing Z alkenes, such as an antioxidant plasmalogen phospholipid the researchers synthesized.
In olefin metathesis chemistry, two carbon-carbon double bonds react to form a new alkene and an alkene by-product, usually ethylene. The reaction has proven so useful that its inventors, including Schrock, garnered the Nobel Prize in Chemistry in 2005. But until now, the only reported instances of Z-selective cross-metathesis—wherein two different alkenes are brought together to make a new Z alkene—involved substrates with an sp-hybridized substituent, such as enynes.
Hoveyda’s group reports the new Z-selective cross-metathesis using either enol ethers or allylic amides as one of the reactants, but Hoveyda says the reaction also works with several other types of alkenes. The molybdenum catalysts that generate the Z alkenes are easy to prepare, relatively inexpensive, and can be fine-tuned by interchanging ligands, he adds.
The arrangement of ligands “allows the catalyst to be highly flexible and adapt to the somewhat severe structural gymnastics” of the olefin metathesis reaction, Hoveyda says. Furthermore, the catalyst’s aryloxide ligand creates a cramped environment in which cis-metallocyclobutane intermediates are preferred, thereby generating exclusively Z products.
The team also performs the reaction at reduced pressure. This removes the ethylene by-product that can occupy the catalyst and convert the Z olefins into E olefins.
“Several aspects of these new metathesis reactions remain to be further refined,” Daesung Lee, a chemistry professor at the University of Illinois, Chicago, writes about the work in a commentary in Nature. “Nevertheless, these Z-selective cross-metathesis reactions are highly promising and will potentially be of use for the preparation of numerous compounds, with far-reaching consequences for the future of metathesis chemistry.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter