Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Water Serves As A Protein Glue
Adhesive water bridges help stabilize hydrophilic protein-protein interactions and guide the molecules together
by Jyllian N. Kemsley
April 11, 2011
| A version of this story appeared in
Volume 89, Issue 15
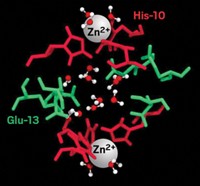
Water forms an adhesive hydrogen-bond network in the interface between hydrophilic protein surfaces, suggests a group led by Volkhard Helms of Saarland University, in Germany (Nat. Commun., DOI: 10.1038/ncomms1258). Hydrophilic protein interactions are not as well studied as hydrophobic protein-protein interactions, in which water molecules are typically excluded from the interface. To learn more, Helms and colleagues turned to a computer model to determine the association between the hydrophilic surfaces of barnase, a bacterial ribonuclease, and its inhibitor protein, barstar. They found that water bridges form between protein residues as the two molecules come together. These bridges stabilize the proteins’ initial interactions and guide them together. Water between the proteins also has a reduced dielectric constant compared with bulk water, a characteristic that appears to enhance electrostatic interactions between the proteins, the researchers note. Water continues to play an adhesive role once the proteins are docked, with the known crystal structure of the complex showing nine water molecules that may mediate hydrogen bonds between the proteins, in addition to direct contacts between amino acids.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter