Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Friedel-Crafts Takes A New Gig
Organic Synthesis: Silane-fueled, proton-catalyzed strategy extends carbon-carbon coupling
by Stephen K. Ritter
May 2, 2011
| A version of this story appeared in
Volume 89, Issue 18
Thanks to the superstrength of the silicon-fluorine bond, chemists at the University of Zurich have developed a new incarnation of the venerable Friedel-Crafts reaction: intramolecular aryl-aryl coupling of fluoroarenes to make custom polycyclic aromatic hydrocarbons.
Friedel-Crafts reactions, a classic approach to forming carbon-carbon bonds, have been around since Charles Friedel and James M. Crafts first reported the chemistry in 1877. The reactions typically use a strong Lewis acid catalyst, such as AlCl3, to couple an aromatic ring and an alkyl halide or acyl halide.
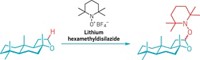
In a new take on the reaction, Oliver Allemann, Jay S. Siegel, and coworkers used a triisopropylsilyl cation as a Lewis acid paired with a carborane anion (Science, DOI: 10.1126/science.1202432). The weakly coordinating carborane anion keeps out of the way, giving the silyl cation freedom to activate the C–F bond of a fluoroarene substrate, Siegel explains. The reaction is driven by the reactants’ thermodynamic urge to trade the strong C–F bond for an even stronger Si–F bond, which forms when the silyl cation abstracts the fluorine. The incipient arene carbocation undergoes intramolecular C–C bond formation, a ring-closing step that creates a polycyclic aromatic hydrocarbon derivative.
The researchers devised a complete catalytic cycle for their reaction by using dimethyldimesitylsilane as the base to neutralize a proton left over from the C–C coupling. This acid-base interaction conveniently shuttles the proton to generate a new silyl cation and innocuous mesitylene by-product.
“Goading an aryl fluoride into a Friedel-Crafts reaction is indeed outside most chemists’ idea of what’s plausible in organic synthesis,” says Oleg V. Ozerov of Texas A&M University, whose lab has explored the utility of silyl carborane catalysts. “This design had to be cleverly tuned to make the reaction catalytic.”
Although the new method provides a novel route to polyaromatic systems, Ozerov says, “the synthetic idea behind it may well have a much broader scope.”
The Zurich chemists tested the reaction on several mono- and difluorinated polycyclic hydrocarbons, generating new five- and six-membered unsaturated rings to form an assortment of fluoranthene, triphenylene, and corannulene derivatives. Still, the reaction can’t form four-membered rings or carry out intermolecular couplings. Siegel says his group is already working to address these lingering limitations.
“These intramolecular arylations represent a powerful new method not only for rapidly extending planar substructures of graphene but also for increasing the curvature and complexity of fullerene substructures,” says Boston College’s Lawrence T. Scott, a polycyclic hydrocarbon expert. “In a year when the Nobel Prize is celebrating the importance of building aryl-aryl bonds by palladium-catalyzed cross-coupling, it’s exciting to see such a completely new method to accomplish the same objective using only main-group elements.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter