Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Jolting The Diels-Alderase Hunt
Biochemistry: Newly discovered enzyme could help determine whether Diels-Alder reaction occurs in nature
by Carmen Drahl
May 9, 2011
| A version of this story appeared in
Volume 89, Issue 19
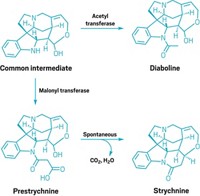
The search may be nearing an end for an enzyme that can catalyze one of chemistry’s most recognizable transformations—the Diels-Alder reaction (Nature, DOI: 10.1038/nature09981). A new study establishes how the insecticide spinosyn A is made in nature and introduces the first enzyme that exclusively catalyzes a [4+2] cycloaddition, a class of reactions that includes the Diels-Alder reaction.
Since its debut 83 years ago, the Diels-Alder reaction, which forms a cyclohexene ring from a conjugated diene and an alkene or alkyne, has become a powerful way to build industrial and pharmaceutical chemicals. For almost as long, researchers have wondered whether nature is also capable of this chemistry. Now, Hung-wen (Ben) Liu and coworkers at the University of Texas, Austin, have found an enzyme called SpnF that might put that question to rest.
SpnF is encoded by one of four genes Liu’s team studied, from a gene cluster that the microbe Saccharopolyspora spinosa uses to make spinosyn A, which Dow Agrosciences markets as an insecticide. Liu’s team purified proteins from each of the four genes and tested their activity on spinosyn precursors. They used nuclear magnetic resonance and mass spectrometry to identify products and high-performance liquid chromatography to track reaction kinetics.
It turned out that SpnF performs one job exclusively: accelerating [4+2] cyclization to form a cyclohexene. The reaction occurs on its own, but with SpnF it takes 20 minutes rather than 2 hours, Liu says. Swift cyclization may keep side products from forming, he explains.
“This enzyme’s the first of its kind,” says John C. Vederas of the University of Alberta, Edmonton. A handful of other proteins, including one his team discovered, have been flagged as likely Diels-Alderases, but all of them have catalyzed other reactions too, Vederas says.
Extra functions make it tough to subject Diels-Alderase contenders to detailed studies of mechanism, so SpnF could offer the clearest answer to whether Diels-Alder reactions occur in nature, writes Georgia Institute of Technology chemist Wendy L. Kelly in a commentary accompanying the study (Nature, DOI: 10.1038/473035a).
Liu says his team hasn’t yet determined SpnF’s mechanism. For SpnF to be a bona fide Diels-Alderase, its mechanism must be concerted—forming two carbon-carbon bonds at the same time with no transient intermediates. “My gut feeling is this is a Diels-Alder reaction,” Liu says. “But we don’t have the evidence yet.” His group is collaborating with an X-ray crystallographer to determine what SpnF looks like and is preparing isotopically labeled versions of spinosyn A precursors for more kinetic studies.
The enzyme’s catalytic activity is low but not unheard of, says Forrest E. Michael, a University of Washington organic chemistry professor who last year helped design an enzyme that catalyzes a Diels-Alder reaction (C&EN, July 19, 2010, page 5). The interest now lies in figuring out how SpnF works, he says. “As synthetic organic chemists, we have many ways of accelerating these kinds of cyclizations. It’ll be exciting to see what nature chose.”





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter