Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Cisplatin To Blame For Zinc Deficiency
When the anticancer drug binds albumin, it blocks the protein’s zinc binding site, causing the chemotherapy side effect
by Stu Borman
May 16, 2011
| A version of this story appeared in
Volume 89, Issue 20
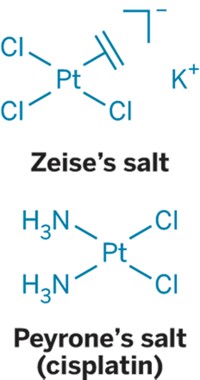
New findings about the way the anticancer drug cisplatin binds to the common blood protein albumin may explain how cisplatin causes zinc deficiency in chemotherapy patients. Cisplatin, which is widely used to treat ovarian, testicular, bladder, head, and other solid tumors, is believed to kill cancer cells primarily by binding to DNA. It also binds to albumin, but the mechanism of that binding interaction has never been fully characterized. Now, a group has used tryptic digestion, liquid chromatography, and tandem mass spectrometry to identify the sites on albumin where cisplatin binds (Chem. Commun., DOI: 10.1039/c1cc11627d). The study was carried out by Fuyi Wang of the Chinese Academy of Sciences, in Beijing; Peter J. Sadler of the University of Warwick, in England; and coworkers. It shows that cisplatin cross-links two key histidines on albumin, thus blocking the protein’s major zinc-binding site. The researchers believe this interaction may account for the zinc deficiency in blood and excessive zinc in urine observed in some cisplatin-treated patients. The work could help lead to cisplatin analogs that lessen or eliminate these side effects.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter