Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Cross-Couplings Go With The Flow
MIT researchers have developed an efficient multistep Suzuki-Miyaura reaction in a continuous-flow microreactor system
by Stephen K. Ritter
June 6, 2011
| A version of this story appeared in
Volume 89, Issue 23

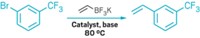
Two MIT research groups have combined their talents to develop an efficient Suzuki-Miyaura cross-coupling reaction in a continuous-flow microreactor system (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201101480). Microreactor expert Klavs F. Jensen’s group and organic chemist Stephen L. Buchwald’s group took on the effort to see how they might develop multistep organic syntheses in a more streamlined manner. Microreactors provide safer operation, better heat and mass transfer, improved control over reactant residence time, more protection from air and moisture, and ease of scale-up than does batch processing, they note. In a test reaction, the MIT researchers first combined a phenol with triflic anhydride in a 100-μL tube reactor to create an aryl triflate intermediate. In the subsequent step, a palladium catalyst in a 400-μL packed-bed reactor mediated coupling of the aryl triflate with an aryl boronate to produce the biaryl product. Key to the success of the synthesis was using a microfluidic liquid-liquid extractor unit to purify the aryl triflates on the fly and using the packed-bed reactor that optimized reagent mixing in the coupling step. The reactions were completed in as little as seven minutes of residence time, the team reports, and produced isolated yields of 95% or better on a millimole-per-hour scale.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter