Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Arsenate-Phosphate Debate Continues
Review suggests controversy should stimulate interest in studies of enzyme promiscuity
by Carmen Drahl
January 17, 2011
| A version of this story appeared in
Volume 89, Issue 3
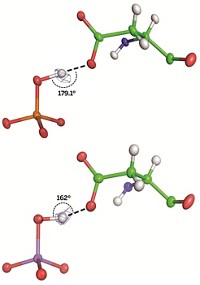
A high-profile claim last month that a microbe could incorporate arsenic into its DNA spawned vociferous criticism (C&EN, Dec. 13, 2010, page 7). Now, Dan S. Tawfik of Israel’s Weizmann Institute of Science and Ronald E. Viola of the University of Toledo have written a review suggesting that even though arsenic’s chemical properties make the claim unlikely, the dispute should spur new arsenic biochemistry studies (Biochemistry, DOI: 10.1021/bi200002a). Not only would arsenic-based DNA be unstable in water, but living things that use arsenate (AsO4 3–) and phosphate (PO4 3–) would also need to evolve enzymes that can tell those two nearly identical species apart, a challenging molecular recognition problem, the authors write. Previous studies suggest that phosphate-utilizing enzymes tend to accept arsenate as an alternative substrate, although the resulting reaction products rapidly decompose. More work must be done to determine whether strict discrimination between arsenate and phosphate is possible and what protein structural features might drive selectivity, Tawfik says. Such research could provide clues about how flexible life’s chemistry can be, Viola says, adding, “we have to keep an open mind about what’s possible.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter