Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Gaseous Carbonic Acid Trapped And Analyzed
Elusive molecule shown to exist in monomeric and dimeric forms
by Mitch Jacoby
January 17, 2011
| A version of this story appeared in
Volume 89, Issue 3
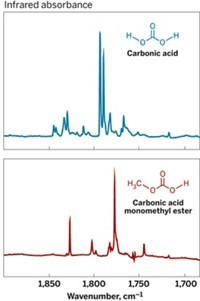
Carbonic acid, an elusive but important compound in geochemical, physiological, and atmospheric processes, has been trapped and analyzed spectroscopically in the gas phase by a team of researchers headed by chemists in Austria (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201004729). H2CO3 plays key roles in regulating blood pH, acidifying oceans, and dissolving minerals. The compound has been studied previously as a solid, but the gaseous species had escaped direct detection because it rapidly decomposes to water and carbon dioxide. That conventional wisdom needs to be updated, according to Thomas Loerting of the University of Innsbruck, Hinrich Grothe of Vienna University of Technology, and coworkers. The team reports that it has trapped gas-phase carbonic acid molecules in a noble-gas matrix at cryogenic temperatures and that the molecule is stable above 200 K. In addition, on the basis of infrared spectroscopy studies, the group proposes that H2CO3 exists as a mixture with a 1:10:1 ratio of two monomeric conformers (cis-trans and cis-cis) and a cyclic dimer, respectively. These results could aid the search for gas-phase carbonic acid in astrophysical environments, the researchers say.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter