Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Single Atoms Mediate Reaction
Catalysis: Isolated platinum atoms remain stable and active in oxidations
by Mitch Jacoby
August 1, 2011
| A version of this story appeared in
Volume 89, Issue 31

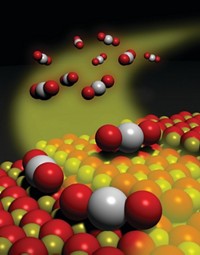
A wet-chemistry method can be used to prepare catalysts featuring single isolated precious-metal atoms supported on a metal oxide surface, according to a research team based in China and the U.S. (Nat. Chem., DOI: 10.1038/nchem.1095). The work may lead to low-cost industrial catalysts and addresses questions in fundamental catalytic science.
Supported catalysts consisting of particles of platinum and other precious metals anchored on oxides are widely used in automotive emissions cleanup and many industrial catalytic processes. Because only the metal atoms at the particle surfaces catalyze reactions, manufacturers aim to make these particles as tiny as possible to maximize metal use. But making uniform subnanometer-sized particles remains challenging. And the tiny particles’ tendency to diffuse and agglomerate deactivates catalysts.
By tuning the temperature, pH, and other parameters in a coprecipitation process, the China-U.S. team has come up with a synthesis that sidesteps those problems. Using atomic-resolution microscopy and other analytical methods, Botao Qiao and Tao Zhang of China’s Dalian Institute of Chemical Physics; Jun Li of Tsinghua University, in Beijing; Jingyue Liu of the University of Missouri, St. Louis; and coworkers have determined that their procedure yields nanocrystallites of iron oxide with isolated platinum atoms dispersed across the surface.
The group compared the single-atom catalyst with other platinum catalysts and with a high-performance gold reference catalyst in CO oxidation reactions. One set of tests evaluated the catalysts’ ability to oxidize CO in the presence of an abundance of hydrogen. The reaction rids hydrogen of low levels of CO, a common contaminant that poisons fuel-cell catalysts. In all cases the researchers found that the single-atom catalyst remains stable under typical reaction conditions and is at least two to three times more active than the other catalysts.
“This is very exciting and timely work,” says Charles H. F. Peden of Pacific Northwest National Laboratory. The catalysis community has been considering whether a single atom can serve as the active site in heterogeneous catalysts, he notes. “This study answers the question with a resounding yes,” he adds.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter