Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Prefab Synthesis Moves Ahead
Organic Chemistry: First stable α–boryl aldehydes ease preparation of complex small molecules
by Stu Borman
August 29, 2011
| A version of this story appeared in
Volume 89, Issue 35
Two studies report the first isolation of α-boryl aldehydes, bifunctional reagents that facilitate synthesis of organic compounds that were previously difficult or impossible to make. The work could enable nonexperts in synthesis to rapidly compose complex organic molecules from simple building blocks in an automated manner—similar to the way peptides are made today.

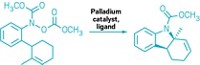
α-Boryl aldehydes are usually extremely unstable, but newly discovered stable ones are now stored in bottles in Andrei K. Yudin’s lab at the University of Toronto and Martin D. Burke’s lab at the University of Illinois, Urbana-Champaign. Zhi He and Yudin and, independently, Junqi Li and Burke discovered the stable α-boryl aldehydes serendipitously while carrying out other reactions, found that they form by a new type of rearrangement, and used them to create numerous organic products (J. Am. Chem. Soc., DOI: 10.1021/ja205910d, 10.1021/ja205912y).
These aldehydes contain two functional groups that can react independently: a nucleophilic boryl carbon and an adjacent electrophilic aldehyde. He and Yudin found that stable α-boryl aldehydes can form when a boryl group on an N-methyliminodiacetic acid (MIDA) boronate epoxide moves from one epoxide carbon to the other and the epoxide opens to form aldehyde.
He and Yudin functionalized the α-boryl aldehydes to form heterocycles, alcohols, olefins, enamines, α-boryl acids, alkenyl triflates, and other compounds, including unnatural amino acids. Most of these products retain the boryl group, which can be further manipulated synthetically.
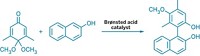
MIDA boronates were developed earlier by Burke’s group, and about 75 of them are commercially available from Sigma-Aldrich as modular agents for coupling reactions. In the new study, Li and Burke developed a chiral version of MIDA—pinene-derived iminodiacetic acid (PIDA). They found that the ligand facilitates stereoselective epoxidation of vinyl boronates and that the resulting PIDA boronate epoxides form chiral α-boryl aldehydes via the same unprecedented epoxide rearrangement.
They used the chiral α-boryl aldehydes to access new bifunctional building blocks that enable iterative stereocontrolled couplings at tetrahedral (sp3) carbon atoms, whereas iterative synthesis with MIDA boronates was previously restricted primarily to couplings of planar (sp2) carbons. They demonstrated the utility of such sp3 couplings by using them to synthesize a glucagon receptor antagonist Merck & Co. is developing for diabetes treatment. Burke believes a collection of perhaps 500 commercial MIDA boronate-type coupling reagents, including PIDA boronates and α-boryl aldehydes, could one day make modular, automated organic synthesis widely available.
“That α-boryl aldehydes are stable is a surprise, and that they can be made easily and in a stereocontrolled fashion is icing on the cake,” comments synthetic organic chemist James P. Morken of Boston College. “The range of transformations already developed by the Burke and Yudin groups clearly shows these building blocks will enable new synthesis strategies,” he remarks.
Asymmetric synthesis specialist Cathleen M. Crudden of Queen’s University, in Kingston, Ontario, adds that “with Burke’s use of chiral versions of MIDA boronates to generate carbon-boron bonds with control of chirality and Yudin’s demonstration of the amazing number of reactions these species can undergo, it is clear these reagents dramatically expand the scope of compounds that can be made using boron chemistry.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter