Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Gold Sulfide’s Diverse Geometry
A computational study has determined that S–Au–S building blocks are key to unique cluster structures
by Mitch Jacoby
February 14, 2011
| A version of this story appeared in
Volume 89, Issue 7
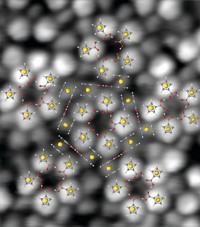
Gold and sulfur assemble into small, stable anionic clusters that are either hollow or contain a single atom in the center, according to theoretical work published in ACS Nano that describes the structures and electronic properties of this class of clusters for the first time (DOI: 10.1021/nn103217z). The study, which focused on gold sulfide clusters containing up to 15 gold atoms, deepens understanding of the composition, stability, and other properties of metal-based clusters. The investigation also aids interpretation of recent results of ion-mobility mass spectrometry experiments indicating that some of the clusters are particularly stable. Yong Pei and Xiao Cheng Zeng of the University of Nebraska, Lincoln, and coworkers found that the high stability of the S–Au–S structural unit supports formation of various hollow clusters, including Au6S4 –, Au9S6 –, and Au12S8 –, which exhibit tetrahedron, triangular prism, and cuboctahedron structures, respectively. The stability of the S–Au–S building motif also leads to clusters with a gold atom encapsulated in a polyhedron such as Au11S6 –, which has triangular prism geometry, as well as clusters such as Au9S5 –, which adopts a unique pyramidal structure.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter