Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Platinum Nanocrystals Show Their Best Face
A novel strontium titanate support material guides crystals to grow with nonstandard geometry
by Mitch Jacoby
February 14, 2011
| A version of this story appeared in
Volume 89, Issue 7

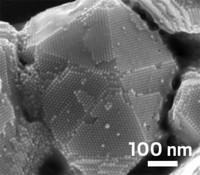
A strategy for synthesizing highly dispersed metal nanoparticles can lead them to adopt a specific orientation on a substrate, which may prove useful for designing nanoparticle catalysts, researchers at Northwestern University report (Nano Lett., DOI: 10.1021/nl104263j). Subtle differences in the structure and electronic properties of one face of a metal crystal relative to another face can lead to measurable differences in the catalytic activity of nanoparticles, even though all of a crystal’s faces consist of atoms of the same element. Scientists have previously tried coaxing metal particles to expose a catalytically preferred crystal face by modifying particle shape and altering synthesis conditions. But upon extended exposure to high temperatures and reactive conditions—the norm for industrial catalysis—particle structures often change, reverting to the most stable and perhaps less catalytically active geometry. Northwestern’s James A. Enterkin, Kenneth R. Poeppelmeier, and Laurence D. Marks have now come up with one way to sidestep this problem. By growing platinum nanoparticles on SrTiO3 “nanocuboids,” a novel high-surface-area material, the team finds that the platinum nanoparticles adopt thermodynamically stable shapes, grow only along a single crystal direction, and expose different faces than they do when supported on common polycrystalline materials such as alumina and silica.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter