Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Catalytic Methanol Coupling Achieved
An iridium complex catalyzes the direct C–H functionalization of methanol, a first for the single-carbon compound
by Stu Borman
February 28, 2011
| A version of this story appeared in
Volume 89, Issue 9
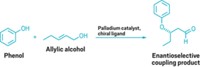
Methanol is a high-volume chemical feedstock and thus a readily available reagent. But its ability to react at carbon rather than oxygen in chemical synthesis is limited—it’s restricted primarily to the Monsanto and Cativa processes for carbonylating methanol to form acetic acid. Michael J. Krische and coworkers of the University of Texas, Austin, have now developed iridium complexes that catalyze the direct C–H functionalization of methanol with allenes to form higher alcohols bearing a quaternary carbon center (Nat. Chem., DOI: 10.1038/nchem.1001). The regioselective hydrohydroxymethylation process does not generate stoichiometric by-products. “Methanol is not an obvious choice of reagent for C–C bond formation, and it is a clever hydrogen-transfer sequence combined with a highly reactive catalyst that has allowed Krische and coworkers to make this significant advance,” comments carbon-coupling expert Jonathan M. J. Williams of the University of Bath, in England. Krische notes that his group hopes to advance the work further by developing “related alcohol C–C couplings applicable to renewable feedstocks, such as the coupling of glycerol to α-olefins.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter