Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
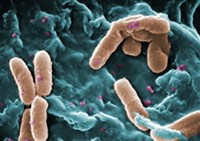
Hitting TB With Toxic Gas
Medicinal Chemistry: Small molecules that release sulfur dioxide could stop the growth of the bacteria that cause tuberculosis
by Katharine Sanderson
December 9, 2011

Small amounts of sulfur dioxide can slow the growth of the bacteria that cause tuberculosis, researchers report (J. Med. Chem., DOI: 10.1021/jm201023g). The toxic gas could form a new avenue of drug research for the disease.

Researchers in India, led by Harinath Chakrapani at the Indian Institute of Science Education and Research Pune, were inspired by a drug in clinical trials for TB. It releases small amounts of nitric oxide, a toxic gas that can have beneficial physiological effects in small doses.
Chakrapani wanted to do the same with another toxic gas, SO2, which finds use as an antibiotic in winemaking. He investigated a family of 2,4-dinitrophenylsulfonamides that can react with thiols to release SO2. “Nobody has really thought of it before,” says Chakrapani.
To test the idea, the researchers added cysteine, a thiol-containing amino acid, to various 2,4-dinitrophenylsulfonamides to see which molecules produced the most SO2. The researchers then added solutions of 11 SO2-producing molecules to growing Mycobacterium tuberculosis colonies. After 28 days, they saw that the molecules that released the most SO2 also were the best at inhibiting the growth of the bacteria. What’s more, the most potent molecule slowed growth at lower concentrations than the standard tuberculosis drug isoniazid did: At 0.37 µM, isoniazid reduced growth by 99%, while the researchers’ compound reached the same level of inhibition at 0.15 µM. To rule out sulfite as the active agent, the researchers tested sulfite ions against the bacteria. Sulfite did not inhibit bacterial growth.
The active molecules are as much as 50 times more toxic to tuberculosis than to human cells, Chakrapani found. Chakrapani now wants to investigate related molecules, because the dinotrophenylsulfonamide structure, he says, “gives us a handle to tune the rate of SO2 generation using very simple structural changes.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter