Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Metal Ions Guide Protein Assembly
Supramolecular Biochemistry: Zinc binding directs proteins to form complex structures
by Stu Borman
March 12, 2012
| A version of this story appeared in
Volume 90, Issue 11

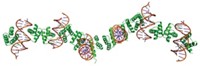
By using metal ions to link protein molecules to one another, researchers have assembled proteins into elaborate and flexibly modifiable sheets, tubes, helical chains, and other objects (Nat. Chem., DOI: 10.1038/nchem.1290).

The approach is unique in its ability to use protein units to construct complex, changeable assemblies in a range of shapes and sizes, say the researchers carrying out the work. Until now, the goal of making such complex protein assemblies has met with only limited success and has been considered largely unachieved.
The new technique could make it possible to design and create polymers with protein-based functions and properties for nanotechnology applications. Its developers also believe it will be useful for learning more about how proteins crystallize.
Inorganic chemist F. Akif Tezcan of the University of California, San Diego, and coworkers used zinc ions to induce proteins with added metal-coordination sites to assemble into nanotubes and crystalline arrays. The structures adopt a variety of shapes and have nanometer to micrometer dimensions.
The metal links between proteins are noncovalent, making the structures flexible. The supramolecular assemblies’ shapes and properties can be tuned by changing metal concentrations, pH levels, and other conditions. Tezcan notes that the assemblies are similar to viral capsids or microtubules, which also change structure in response to metal binding, chelation, or pH changes.
During the past decade, several groups have used techniques such as genetic engineering of fusion proteins and biotin-streptavidin binding to create protein assemblies. The resulting structures were relatively simple because interactions between large, proximate protein surfaces are generally hard to control and program. In addition, previous assemblies “lacked substantial order or were not responsive to chemical stimuli,” Tezcan says.
Tezcan and coworkers put two or three strategic metal-coordination sites (such as bis-histidine motifs) in each protein and engineer the protein’s surface to better orient the sites. When they add zinc ions to the modified protein, supramolecular protein nanotubes and crystalline arrays form.
The protein science community is abuzz with rumors that several research groups are about to report complex protein assemblies formed by other means. The Tezcan group’s strategy may thus be the first of several advances in supramolecular protein design.
Todd O. Yeates of the University of California, Los Angeles, whose group pioneered supramolecular protein design in 2001 by using gene fusion to engineer protein cages and filaments, comments that the study “is very nice work, to be sure. It’s unique among studies that have been done before in the use of metal coordination, which appears to be turning out to be quite a versatile approach.”
Biochemical nanotechnologist Chengde Mao of Purdue University says using metals to create protein assemblies “is easier to implement and more orientationally controllable” than earlier strategies. Applications include using metal-linked protein arrays as templates for nanofabrication and as a means to organize other biomacromolecules for structure determination, he says.
Northwestern University’s Chad A. Mirkin, who specializes in supramolecular coordination and DNA assembly, calls the study “an elegant, tour de force demonstration of the power of protein engineering and coordination chemistry.” It could help create a new subfield in soft-matter materials science, he adds. “I think Tezcan and coworkers are really onto something here, Mirkin adds.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter