Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Common Antibiotic Mechanism Shown
Microbiology: Antibiotics kill bacteria by oxidizing guanine nucleotides
by Celia Henry Arnaud
April 23, 2012
| A version of this story appeared in
Volume 90, Issue 17
Guanine oxidation is at the heart of the cell-killing abilities of many common antibiotics, biologists at MIT and Boston University report (Science, DOI: 10.1126/science.1219192). The findings, if confirmed, could improve scientists’ understanding of the way existing antibiotics actually work.
Previously, James J. Collins and coworkers at Boston University reported that diverse antibiotics with different targets have a common cell-killing mechanism (C&EN, Sept. 10, 2007, page 8; Cell, DOI: 10.1016/j.cell.2007.06.049). They found that various antibiotics trigger the production of hydroxyl radicals, leading to oxidative damage and cell death, but they didn’t describe the process in detail.
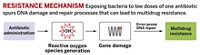
Now, Graham C. Walker of MIT and coworkers have teamed up with Collins’ group to show that a specific type of oxidative damage, guanine oxidation, is the main cause of cell death by bactericidal antibiotics. In this process, hydroxyl radicals oxidize the DNA base guanine to form 7,8-dihydro-8-oxoguanine, or 8-oxoguanine.
Cells deal with 8-oxoguanine by excising and replacing the base in DNA, but those defenses can be overwhelmed by too much 8-oxoguanine. If the modified bases occur too often and too close together, the repair mechanism instead breaks the DNA strand. The accumulation of these breaks leads to cell death.
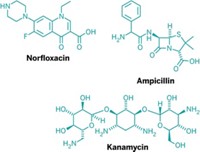
Walker and Collins’ team treated Escherichia coli cells with different classes of antibiotics: a β-lactam (ampicillin), a quinolone (norfloxacin), and an aminoglycoside (kanamycin). By adjusting the expression of enzymes in cells’ guanine oxidation defense mechanism, the researchers showed that the bacteria became less sensitive to antibiotics when expression increased and more sensitive when expression decreased.
“Hydroxyl radicals are not only extremely reactive but also very nondiscriminatory in their reactions,” Walker says. “There was no particular a priori reason to suspect that oxidation of one very particular type of biomolecule was particularly responsible for cell death.”
The findings suggest that the usefulness of current drugs to treat bacterial infections could be extended with adjuvants that target the guanine oxidation pathway, the researchers write.
“The work is certainly interesting and thought-provoking,” says Shahriar Mobashery, an antibiotics expert at the University of Notre Dame. “No doubt its generality with other antibiotics, beyond the three classes that have been looked at, will be investigated in the coming weeks and months.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter