Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Fluorine-Bond Donors Escort Anions Past Membranes
Halogen bonds help transport anions across cellular lipid bilayers
by Elizabeth K. Wilson
June 25, 2012
| A version of this story appeared in
Volume 90, Issue 26
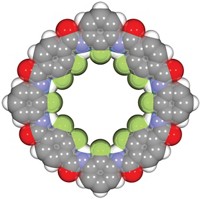
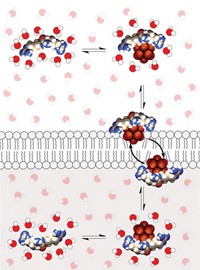
Small, perfluorinated molecules with the ability to form halogen bonds can help transport anions across the lipid bilayer membrane of cells—a complex task that usually requires the concerted actions of large proteins, report Stefan Matile of the University of Geneva and colleagues (Nat. Commun., DOI: 10.1038/ncomms1902). Such effective, small transport systems have numerous potential applications, including drug delivery and sensors. Researchers have previously attempted to use small molecules such as detergents to transport ions, but these efforts have caused membrane destruction and content leakage. Matile’s group found, however, that molecules such as perfluoroiodohexane and tetrafluorodiiodobenzenes surround chloride and hydroxide ions, forming strong, directional halogen bonds. The halogen-bonded complexes then slip across lipid bilayer membranes selectively. Matile and coworkers note that one of these molecules, trifluoroiodomethane, contains only one carbon and therefore represents the smallest possible version of the organic ion transporters.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter