Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Cut-And-Sew Route Makes Fused Rings
Rhodium-catalyzed method overturns typical selectivity for C–C bond activation
by Carmen Drahl
July 2, 2012
| A version of this story appeared in
Volume 90, Issue 27
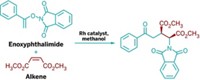
With help from a chelating ligand, chemists have found an efficient new route to fused-ring systems similar to those in alkaloid drugs such as morphine or codeine (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201202771). The work also presents a new twist on carbon-carbon bond activation. Chemists typically forge alkaloid core structures with lengthy multistep syntheses. Now, assistant professor Guangbin Dong and postdoctoral researcher Tao Xu have stitched the three-ring cores together in one rhodium-catalyzed step. The pair, from the University of Texas, Austin, began with benzocyclobutenones. They had to avoid cleaving their substrate’s most reactive C–C bond, and break a less reactive C–C bond instead. They learned that 1,1-bis(diphenylphosphino)butane ligands helped achieve that selectivity. They think an olefin in the substrate also guides the reaction, which requires zinc and rhodium in tough cases. “While the fundamental metal-catalyzed approach to multiring systems using four-membered ring strain is not without precedent, the authors have been creative and insightful in their chosen, and nontrivial, application,” says organometallic chemist Lanny S. Liebeskind of Emory University. He looks forward to reading about gentler reaction conditions and a wider array of substrates in follow-up work.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter