Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Making Marinomycin A
Salicylate switch guides total synthesis
by Bethany Halford
July 2, 2012
| A version of this story appeared in
Volume 90, Issue 27
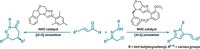
With its power to fight both methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium, the macrodiolide antibiotic marinomycin A is a worthy target for total synthesis. But it’s really the molecule’s symmetrical structure that makes it something of a siren song to synthetic chemists: Its simplicity beckons, only to confound efforts to couple the compound’s two halves. Rather than form the desired 44-membered macrocycle, the polyketide chains tend to cyclize intramolecularly, forming a macrolide rather than the desired macrodiolide. Chemists led by P. Andrew Evans of England’s University of Liverpool have now successfully navigated the synthesis of this compound, using its salicylate moieties as molecular switches. Depending on how the salicylate is functionalized, it can mediate the reactivity of its ester group, either by deactivating it or by making it electrophilic. The salicylate, Evans points out, reduces the electrophilicity of the aryl ester through an intramolecular hydrogen bond, essentially making the group function as an amide in terms of electronics and conformation. “This strategy raises new questions regarding the biosynthetic role of the salicylate and its potential impact on the mechanism of action of these types of agents,” the researchers note in their paper (Nat. Chem., DOI: 10.1038/nchem.1330).




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter